Insights / Blog / RTSM
A Guide to Selecting the Right Randomization and Trial Supply Management (RTSM) System
- Abriti Rai
- June 17, 2024

On this Page
- Summary
- What Is RTSM in Clinical Trials?
- A Brief Evolution of RTSM Systems
- Core Components of an RTSM System
- Types of Randomization in RTSM Systems for Clinical Trials
- What Are the Benefits of Using an RTSM System
- How to Choose the Right RTSM System
- How to Choose the Right RTSM Vendor: Questions to Consider
- RTSM Best Practices and Compliance
- Conclusion
- External References
- Summary
- What Is RTSM in Clinical Trials?
- A Brief Evolution of RTSM Systems
- Core Components of an RTSM System
- Types of Randomization in RTSM Systems for Clinical Trials
- What Are the Benefits of Using an RTSM System
- How to Choose the Right RTSM System
- How to Choose the Right RTSM Vendor: Questions to Consider
- RTSM Best Practices and Compliance
- Conclusion
- External References
Summary
Randomization and Trial Supply Management (RTSM) streamlines clinical research by automating patient randomization and managing the logistics of investigational products. Modern RTSM systems enhance accuracy, improve compliance, and provide study teams with real-time visibility to run faster, more adaptive, and patient-focused trials.
Legacy Randomization and Trial Supply Management (RTSM) systems often take several weeks to months to implement mid-study changes, causing delays that can stall patient enrollment, disrupt drug shipments, and cost sponsors hundreds of thousands of dollars per week. In an industry where every day of delay directly affects both trial budgets and patient outcomes, the need for agile, integrated, and data-driven RTSM platforms has become urgent.
Modern RTSM systems automate everything from patient randomization to drug supply management, giving clinical operations teams the visibility and control they need to run faster, more compliant, and more efficient studies.
What Is RTSM in Clinical Trials?
Randomization and Trial Supply Management (RTSM) is a specialized technology used in clinical research to automate two essential processes:
- Patient randomization: assigning participants to treatment arms without bias.
- Trial supply management: ensuring the right investigational product (IP) is available, shipped, and dispensed accurately at every site.
By combining these functions in a single, centralized platform, RTSM systems reduce manual errors, streamline logistics, and ensure that sponsors, CROs, and sites work from synchronized, real-time data.
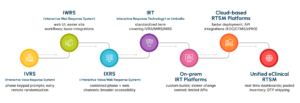
A Brief Evolution of RTSM Systems
RTSM has evolved significantly over the years, from manual randomization methods and early phone-based IVRS systems to today’s unified, cloud-enabled platforms that support global, data-driven trials.
Core Components of an RTSM System
Modern RTSM platforms serve as the operational backbone of clinical trials. At their core, they perform three critical functions: participant management, randomization management, and trial supply management.
Participant Management
An RTSM system tracks every participant’s progress through the trial. It confirms eligibility, assigns visits, captures demographic data, and issues correct dosing instructions automatically. This reduces site burden and human error while maintaining protocol compliance.
Randomization Management
RTSM platforms automate the randomization process, from defining allocation ratios to managing re-randomization when required. They maintain balanced cohorts, eliminate manual bias, and generate audit-ready records. Real-time notifications keep site staff and data teams aligned.
Trial Supply Management
RTSM automates IP tracking from depot to patient, monitors expiry dates, and triggers resupply orders as inventory levels drop. It helps ensure the right drug is at the right site at the right time, with complete visibility across global operations.
Types of Randomization in RTSM Systems for Clinical Trials
Randomization is the foundation of unbiased clinical research. Different studies require different randomization strategies, and modern RTSM platforms can support them all.
Method | Description | Typical Use Case |
Simple Randomization | Each participant is randomly assigned to a treatment group without restrictions. | Small or balanced trials where participant characteristics are evenly distributed. |
Block Randomization | Participants are assigned in fixed-size blocks to maintain equal allocation. | Multicenter studies to ensure balance within each site or phase. |
Stratified Randomization | Participants are grouped (e.g., age, gender, disease stage) before assignment. | Trials where subgroup balance affects outcomes or statistical power. |
Adaptive Randomization | Allocation probabilities change based on interim results. | Data-driven trials that adjust treatment arms in near real time for efficiency or ethical reasons. |
By automating these methodologies, RTSM ensures reproducibility and traceability, which are vital for regulatory validation and statistical accuracy.
What Are the Benefits of Using an RTSM System
A modern RTSM impacts nearly every aspect of execution.
Operational efficiency: Automation eliminates repetitive logistics tasks and minimizes site workload, allowing teams to focus on patient care and protocol adherence.
Data accuracy: Integration with systems like EDC and CTMS removes double entry and reduces reconciliation errors, producing consistent, validated datasets.
Cost control: Predictive resupply and inventory visibility minimize drug overage and waste, which are major hidden costs in trials.
Regulatory readiness: Audit trails, access controls, and automated reporting support compliance with 21 CFR Part 11, GDPR, and other global standards.
How to Choose the Right RTSM System
Selecting an RTSM platform isn’t just about software; it’s about choosing a long-term partner that understands your study design and operational goals. When evaluating potential RTSM vendors, look for these capabilities:
Rapid Deployment
An RTSM system that takes weeks to deploy isn’t ideal for the current age of clinical trials. The ideal RTSM should be deployable within days, allowing trials to start promptly and run smoothly. Quick deployment is essential for maintaining project timelines, reducing costs, and ensuring trials start promptly.
100% Configurable System
The ideal RTSM system should be fully configurable and intuitive. Configurable systems can easily accommodate protocol amendments, various randomization schemes, and diverse trial designs by eliminating the need for complex coding and significantly reducing setup time.
Powerful Integration
A robust RTSM system should offer powerful integration capabilities to ensure seamless interoperability with clinical trial systems like EDC, CTMS, and ePRO. These integrations allow real-time data transfer across systems, minimizing errors, eliminating the need for data reconciliation, enhancing data accuracy, and optimizing operational efficiency.
Adaptive Trial Facilitation
When selecting an RTSM system, prioritize one that supports the dynamic needs of adaptive trials. An ideal RTSM should enable mid-study changes with ease, such as modifying randomization procedures, subject replacement, dosage modifications, sample size adjustments, and trial design alterations.
Additionally, in emergencies, the system should offer support for unblinding to maintain the trial’s integrity and continuity. A well-equipped RTSM system with robust adaptive trial capabilities ensures greater flexibility, allowing clinical trials to navigate evolving requirements and achieve reliable, efficient outcomes.
Support Complex Protocols
An RTSM system must accommodate various study designs, including crossover, sequencing, and extension studies, ensuring it meets the unique needs of each trial. It should also offer robust cohort management capabilities and a range of randomization options, such as static, stratified, dynamic, forced, and adaptive, to provide tailored support for complex trial protocols. This ensures the system can maintain trial integrity and deliver accurate, reliable results.
IP Monitoring and Tracking
The RTSM system should provide comprehensive monitoring and tracking of investigational product (IP) inventory. It is essential for strategic planning and distribution, ensuring inventory levels at each trial site remain adequate. The ability to track patient registrations and IP quantities across trial centers to prevent shortages is also important.
Additionally, the RTSM should promptly alert when inventory levels fall below a threshold, facilitating timely restocking from the depot. This ensures smooth trial operations and minimizes disruptions in treatment delivery.
Adherence to Regulatory Standards and Guidelines
The RTSM system that you are choosing should comply with industry standards such as 21 CFR Part 11, GDPR, and EU Annex 11. If it’s being operated in multiple countries, then the system should comply with the local regulations as well. Also, the data security should be robust, and the system should have a disaster recovery plan in place to ensure the continuation of trials even under unforeseen circumstances.
How to Choose the Right RTSM Vendor: Questions to Consider
- How long does your system take to deploy from configuration to go-live?
- How do you handle mid-study protocol changes?
- What integrations are supported out of the box (EDC, logistics, ePRO)?
- How do you ensure system validation and regulatory compliance?
- What level of customization and post-launch support do you offer?
- Can your system accommodate adaptive trial designs and global-scale studies?
Implementing RTSM in Clinical Trials
Once a vendor is selected, successful implementation depends on clear planning, collaboration, and continuous validation.
- Requirements gathering: Define trial objectives, randomization schemes, visit schedules, and IP flow. Involve data management, supply chain, and regulatory teams from the start.
- System configuration: Build and test the RTSM according to the approved protocol, ensuring every setup aligns with study needs.
- Validation and UAT: Conduct user acceptance testing to confirm reliability and compliance before launch.
- Go-live: Deploy the system, monitor initial performance, and gather feedback for quick adjustments.
Ongoing support: Maintain data quality, manage protocol amendments, and monitor supply performance throughout the trial.
RTSM Best Practices and Compliance
Compliance is central to any RTSM. Every function, from user access to data transmission, must align with global regulatory expectations.
Key best practices
- Validate software changes under GAMP 5 guidelines.
- Maintain secure audit trails and role-based access controls.
- Integrate with other validated systems (EDC, ePRO) for seamless data flow.
- Ensure robust disaster recovery and data retention policies.
Primary compliance standards
Regulation | Focus | RTSM relevance |
Electronic records and signatures | Ensures traceability, audit readiness, and e-signing. | |
Computerized system controls | Requires validation and secure data handling. | |
Data privacy | Protects sensitive participant and site data globally. |
Conclusion
RTSM is evolving in step with broader digital transformation in clinical research. Advanced AI and predictive analytics enable precise forecasting of drug usage patterns, helping sponsors optimize production schedules and reduce costly overage. Cloud-native architectures facilitate faster setup, effortless scaling, and enhanced collaboration across sites. Integration with decentralized clinical trials supports direct-to-patient drug delivery and hybrid models. Emerging machine-learning approaches predict enrollment trends and automate near real-time supply planning.
Together, these innovations are shaping an RTSM ecosystem that is not only reactive but increasingly predictive and proactive, aligned with the future of connected, patient-centric clinical trials.
Clinion’s RTSM
Clinion’s RTSM addresses these challenges directly. Built for rapid deployment and seamless integration, it empowers researchers to navigate trial complexity with confidence. The system offers the flexibility to handle intricate studies while safeguarding data integrity and optimizing resource management. By partnering with Clinion, you can advance research with high standards and speed the path to reliable medical breakthroughs.
External References

Abriti Rai writes on the intersection of AI, automation, and clinical research. At Clinion, she develops content that simplifies complex innovations and highlights how technology is shaping the next generation of data-driven clinical trials.
FAQS
Frequently Asked Questions
RTSM can integrate directly with platforms like EDC, CTMS, and ePRO through secure APIs. This connection allows automatic data updates on patient enrollment, dosing, and supply levels, helping study teams avoid duplicate entries and maintain a unified trial database.
A frequent mistake is treating RTSM setup as a technical step rather than a strategic process. Lack of cross-functional input from data, supply chain, and clinical operations teams often leads to configuration gaps or delays. Early alignment across departments prevents rework and system downtime.
By automating randomization and inventory management, RTSM minimizes drug overage, reduces shipping errors, and shortens study timelines. The system also improves resource allocation by forecasting supply needs and cutting down on manual monitoring time, which directly lowers operational costs.
Most RTSM platforms are intuitive and require minimal technical knowledge. However, site teams benefit from short onboarding sessions focused on randomization workflows, inventory updates, and troubleshooting. Ongoing refresher training ensures consistent accuracy throughout the study.
Yes. RTSM configurations can be tailored for oncology, rare diseases, vaccine studies, and more. Parameters like cohort structures, dosing regimens, and patient visit schedules can be customized to match protocol-specific requirements without major redevelopment.
Platforms that meet these guidelines are validated, secure, and ready for use in global, audit-ready clinical research.
Modern RTSM platforms use encryption, role-based access, and audit trails to protect sensitive data. Cloud-hosted systems comply with security frameworks such as ISO 27001 and GDPR, ensuring both privacy and traceability for all study records.
RTSM is evolving toward predictive, AI-driven systems that can forecast enrollment trends and supply needs. Integration with decentralized trial models and patient-centric delivery options will make future RTSM tools faster, smarter, and more responsive.
Clinion RTSM combines rapid deployment, complete configurability, and seamless integration across eClinical tools. It supports complex adaptive trials, provides real-time visibility, and ensures compliance with global standards, helping sponsors run smarter and more efficient studies.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


