Insights / Blog / RTSM
IWRS/IRT/RTSM: Are they different or the same?
- Rajesh S Pothula
- October 17, 2022

On this Page
- Summary
- The Evolution of Interactive Response Technology in Clinical Trials
- What Is IWRS (Interactive Web Response System)?
- What Is RTSM (Randomization and Trial Supply Management)?
- What Is IRT (Interactive Response Technology)?
- Evolution of Randomization Systems in Clinical Trials
- Why the Industry Moved from IWRS to RTSM
- Core Components of a Robust RTSM Solution
- Step-by-Step IWRS/RTSM Workflow
- Choosing Between IWRS and RTSM
- Vendor Evaluation Checklist for IWRS/RTSM Systems
- Implementation Best Practices for IWRS/RTSM Systems
- Common Challenges and How to Overcome Them
- The Role of AI and Automation in the Future of RTSM
- Conclusion
- External References
- Summary
- The Evolution of Interactive Response Technology in Clinical Trials
- What Is IWRS (Interactive Web Response System)?
- What Is RTSM (Randomization and Trial Supply Management)?
- What Is IRT (Interactive Response Technology)?
- Evolution of Randomization Systems in Clinical Trials
- Why the Industry Moved from IWRS to RTSM
- Core Components of a Robust RTSM Solution
- Step-by-Step IWRS/RTSM Workflow
- Choosing Between IWRS and RTSM
- Vendor Evaluation Checklist for IWRS/RTSM Systems
- Implementation Best Practices for IWRS/RTSM Systems
- Common Challenges and How to Overcome Them
- The Role of AI and Automation in the Future of RTSM
- Conclusion
- External References
Summary
IWRS and RTSM are key technologies in clinical trials, used for patient randomization and trial supply management. RTSM represents the evolution of earlier systems like IVRS and IWRS, offering advanced automation, predictive resupply, and integration with EDC and CTMS platforms to enhance accuracy, efficiency, and compliance in modern adaptive and decentralized studies.
Modern clinical trials rely on a range of digital systems to manage patient randomization, drug supply, and study logistics. Terms like IVRS, IWRS, IXRS, IRT, and RTSM are often used interchangeably, leading to confusion about their distinct roles. While each represents a stage in the evolution of clinical trial technology, Randomization and Trial Supply Management (RTSM) has become the foundation for modern, adaptive trials.
This article demystifies these terms, exploring the key differences between IWRS vs RTSM, their relationship with IRT, and what to consider when choosing systems that ensure data integrity, compliance, and efficiency.
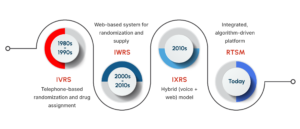
The Evolution of Interactive Response Technology in Clinical Trials
The 1980s marked the rise of Interactive Voice Response Systems (IVRS), which enabled subject randomization via telephone prompts. With the growth of internet infrastructure in the 2000s, Interactive Web Response Systems (IWRS) replaced phone-based processes with browser-based interfaces. Soon after, IXRS introduced hybrid capabilities, supporting both voice and web transactions.
As global trial networks expanded, these systems converged into RTSM platforms, integrating adaptive randomization algorithms, predictive drug supply management, and interoperability with Electronic Data Capture (EDC) solutions. The industry’s focus has since shifted from how sites interact with systems to how effectively those systems manage trial operations.
What Is IWRS (Interactive Web Response System)?
An Interactive Web Response System (IWRS) is a web-based application that enables study teams to randomize patients, manage investigational product (IP) supplies, and track study activity in real time. It acts as a digital bridge between sponsors, CROs, and site personnel, allowing for accurate enrollment, randomization, and drug dispensation through a secure online interface.
Core Features of IWRS
- Patient randomization using predefined algorithms such as simple, stratified, or block designs.
- Drug supply tracking with full accountability across all participating sites.
- Seamless integration with Electronic Data Capture (EDC) systems to enable faster access and data reconciliation.
- Dosing schedule and visit management to maintain protocol compliance and patient adherence.
Use Case: IWRS is best suited for small to mid-sized clinical trials that require reliable randomization and supply oversight without the complexity of adaptive designs or global scalability needs.
What Is RTSM (Randomization and Trial Supply Management)?
RTSM is an evolution of IWRS; it brings together patient randomization with advanced supply chain management, optimizing drug allocation, reducing waste, and ensuring blinding integrity.
Core Capabilities of RTSM
- Advanced randomization algorithms, including minimization, dynamic allocation, and cohort management, to support complex trial designs.
- Predictive supply management and just-in-time (JIT) resupply logic to prevent over- or under-stocking.
- Temperature monitoring and excursion tracking for cold-chain compliance.
- Built-in flexibility to accommodate mid-study protocol amendments and adaptive design changes.
- Comprehensive integration with systems like EDC, CTMS, eCOA, and other eClinical solutions for end-to-end visibility.
In essence, IWRS manages execution at the site level, while RTSM delivers strategic optimization across the entire clinical supply chain.
What Is IRT (Interactive Response Technology)?
RTSM is an evolution of IWRS; it brings together patient randomization with advanced supply chain management, optimizing drug allocation, reducing waste, and ensuring blinding integrity.
Key Functions of IRT in Clinical Trials
- Automates randomization and IP allocation, ensuring accuracy and compliance.
- Maintains patient blinding and balanced treatment group assignments.
- Provides electronic audit trails for full traceability and compliance with FDA 21 CFR Part 11 and EMA Annex 11.
- Enables real-time oversight for sponsors, CROs, and supply chain managers across all study sites.
Summary: Every RTSM solution operates as an IRT system, but not every IRT platform meets the advanced integration and optimization standards of modern RTSM.
Evolution of Randomization Systems in Clinical Trials
Why the Industry Moved from IWRS to RTSM
As clinical trials evolved beyond simple randomization needs, the limitations of standalone IWRS systems became evident. The shift from IWRS to RTSM was driven by the growing complexity of clinical trials. As studies became more adaptive and decentralized, sponsors needed unified systems that could manage randomization, supply forecasting, and compliance efficiently.
From Modality to Functionality
RTSM reframed IRT systems to focus on outcomes, synchronized randomization, real-time supply tracking, and efficient oversight, rather than on the interaction method (voice or web).
Compliance and Regulatory Alignment
Modern RTSM solutions ensure FDA 21 CFR Part 11, EMA Annex 11, and GCP compliance, providing secure audit trails, validation protocols, and system traceability.
Integration with eClinical Ecosystems
RTSM acts as the hub between EDC, CTMS, and other systems, supporting seamless data exchange, fewer handoffs, and enhanced visibility for sponsors.
Core Components of a Robust RTSM Solution
A modern Randomization and Trial Supply Management (RTSM) system does more than randomize patients or track supplies; it connects every part of a trial into one cohesive, intelligent workflow. Below are the essential capabilities that define an effective RTSM platform.
1. Patient Cohort & Randomization Management
A robust RTSM enables fair and flexible patient allocation using algorithms such as minimization, stratified, or dynamic randomization. It supports adaptive designs, multiple cohorts, and protocol-driven logic, helping study teams maintain balance across treatment arms while accommodating mid-study changes.
2. Supply Chain Optimization
An advanced RTSM monitors inventory levels, expiry dates, and temperature conditions in real time. Predictive resupply ensures sites always have sufficient stock, minimizing waste and preventing shipment delays. By automating these logistics, the system helps maintain trial continuity and product integrity.
3. Site & Subject Management
RTSM systems simplify everyday site operations, from screening and enrollment to visit tracking and dosing schedules. Multilingual and mobile-friendly access ensures that teams across different geographies can manage activities consistently and accurately, supporting both centralized and decentralized trials.
4. Rapid Deployment
In fast-moving studies, time is critical. The ideal RTSM should be deployable within days, not weeks, allowing trials to start on schedule. Quick setup reduces delays, lowers costs, and keeps studies aligned with regulatory and operational timelines.
5. Configurable and Intuitive Design
A configurable RTSM lets teams tailor system logic (randomization methods, supply rules, visit plans) without custom coding. This adaptability is invaluable for handling protocol amendments or evolving trial needs, allowing updates to be implemented quickly while keeping operations uninterrupted.
6. Seamless Integration
Modern RTSM platforms integrate smoothly with EDC, CTMS, ePRO, and eCOA systems to ensure data consistency across platforms. These integrations eliminate duplicate entries and reconciliation efforts, enabling real-time information flow that improves accuracy and operational efficiency.
7. Adaptive Trial Support
Adaptive designs demand systems that evolve with the study. A capable RTSM supports mid-study adjustments, such as randomization ratio changes, cohort additions, or dosage modifications. It also allows for controlled unblinding in emergencies, maintaining compliance and protecting trial integrity.
8. Support for Complex Protocols
Every clinical trial is different. A strong RTSM can handle crossover, sequencing, or multi-arm studies and manage multiple randomization strategies. Its flexibility ensures complex protocols run smoothly without compromising data integrity or study blinding.
9. IP Tracking and Monitoring
Continuous visibility into investigational product (IP) movement is essential. The RTSM should track quantities at depots and sites, monitor patient-level dispensation, and trigger alerts when stock runs low. This proactive monitoring prevents shortages and ensures uninterrupted treatment.
10. Built-in Compliance and Data Security
A reliable RTSM aligns with international regulations like FDA 21 CFR Part 11, EMA Annex 11, and GDPR. Strong data encryption, audit trails, and disaster recovery measures protect both patient information and study continuity, ensuring the system remains secure and compliant across regions.
Step-by-Step IWRS/RTSM Workflow
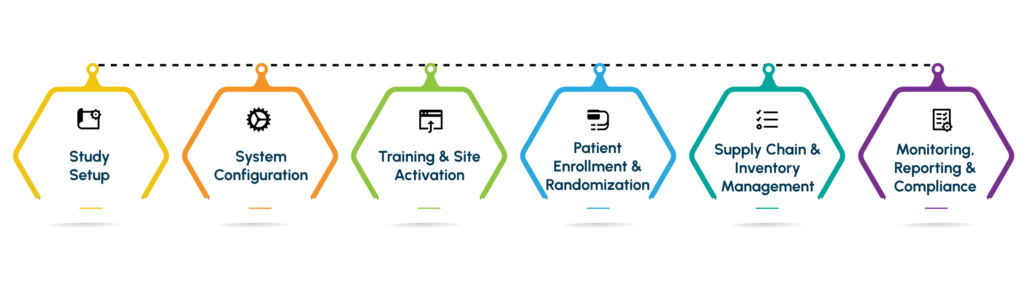
Study Setup
- Define study protocol parameters, treatment arms, visit schedules, and randomization schema.
- Align system configuration with master protocol and pharmacy manuals.
System Configuration
- Configure randomization algorithms (simple, stratified, block, minimization).
- Set up predictive models for supply forecasting and site allocations.
- Establish integration points with EDC, CTMS, eCOA, and other eClinical systems.
- Plan for compliance with FDA 21 CFR Part 11, EMA Annex 11, and GCP guidelines.
Training & Site Activation
- Assign user roles and permissions by site and user type.
- Conduct comprehensive training sessions using sandbox environments.
- Validate workflows, test integrations, and confirm helpdesk readiness.
Patient Enrollment and Randomization
- Register subjects with demographic and visit information.
- Automatically randomize patients to treatment arms using preconfigured algorithms.
- Assign drug kits or investigational products based on randomization output.
- Maintain study blind and ensure audit trail capture.
Supply Chain & Inventory Management
- Track kit inventory, shipments, receipts, and returns in real time.
- Monitor temperature and environmental conditions for cold chain compliance.
- Automate reorder triggers and just-in-time resupply workflows.
- Manage drug accountability and reconciliation for regulatory audits.
Monitoring, Reporting & Compliance
- Access real-time dashboards on enrollment, drug usage, and supply status.
- Handle protocol amendments with system updates and versioning.
- Maintain audit trails and comprehensive documentation for inspections.
- Provide support for emergency unblinding and mid-study adjustments.
Choosing Between IWRS and RTSM
Criteria | When IWRS Fits Best | When RTSM Is Ideal |
Trial Size & Scope | Small or single-country studies | Multi-region, adaptive, or decentralized trials |
Trial Complexity | Fixed dosing or simple trial designs | Complex dosing regimens and temperature-sensitive supplies |
Supply Chain Complexity | Minimal drug supply complexity and logistics | Real-time drug supply reporting, forecasting, and interventions |
Randomization Needs | Basic randomization schema | Advanced randomization methods, adaptive designs |
Integration Requirements | Limited or no integration with other systems | Seamless integration with EDC, CTMS, and eClinical platforms |
System Flexibility | Basic configuration | High flexibility for mid-study protocol changes |
Compliance & Validation | Standard compliance | Full 21 CFR Part 11, GxP, and global regulations compliance |
Reporting & Monitoring | Basic monitoring | Real-time dashboards and predictive analytics |
Vendor Evaluation Checklist for IWRS/RTSM Systems
1. Compliance and Validation
- Must be validated for 21 CFR Part 11, GxP, and other applicable regulations such as EMA Annex 11 and GDPR.
- Maintain complete and traceable audit trails to ensure inspection readiness and regulatory compliance
2. Technical Support and Infrastructure
- Provide secure, redundant global data hosting with built-in backup and disaster recovery.
- Offer 24/7 multilingual technical support and a responsive helpdesk to assist global users.
3. Integration and Interoperability
- Seamlessly integrate with EDC, CTMS, eCOA, ePRO, and other eClinical systems.
- Support real-time data synchronization to reduce manual handoffs and data discrepancies.
4. Architecture and Scalability
- Feature a scalable, modular design that accommodates varying trial sizes, therapeutic areas, and complexities.
- Offer flexibility for both cloud-based and on-premises deployments based on sponsor preferences and compliance needs.
5. System Flexibility and Configurability
- Enable mid-study protocol amendments, adaptive trial designs, and changes to randomization or supply parameters without disruption.
- Provide configurable workflows and study settings without requiring extensive custom development.
6. Vendor Experience and Quality
- Demonstrate a proven track record with studies of similar phase, scale, and geographic reach.
- Supply case studies or references that validate delivery timelines, reliability, and overall quality performance.
7. Security and Data Privacy
- Implement robust role-based access controls, unique user credentials, and end-to-end encryption.
- Ensure full adherence to data privacy laws (e.g., GDPR) and maintain documented security protocols for system and data protection.
Implementation Best Practices for IWRS/RTSM Systems
A successful IWRS/RTSM implementation depends on early alignment, cross-functional collaboration, clear processes, and proactive planning. The following best practices help ensure a smooth rollout and sustained efficiency throughout the trial.
- Engage stakeholders early: Involve clinical operations, IT, biostats, and QA teams from the start to align on goals and compliance needs.
- Define workflows clearly: Map how the system connects with EDC, CTMS, and other tools to avoid data gaps and confusion.
- Plan for Growth: Choose a system that scales easily and supports multiple languages for global studies.
- Validate before launch: Test under GCP and 21 CFR Part 11 standards to confirm accuracy and security.
- Train and support users: Provide regular training and quick help access to ensure confident, consistent use across all sites.
Common Challenges and How to Overcome Them
Even with robust systems, clinical trials face common operational challenges. A well-designed IWRS/RTSM can address these effectively when configured and managed correctly.
Challenge | Recommended Solution |
Overstocking of drugs | Use predictive resupply algorithms to manage inventory levels efficiently and prevent overstocking. |
Data silos | Integrate RTSM early with EDC, CTMS, and other systems to ensure consistent data flow and eliminate duplication. |
Low site adoption | Offer user-friendly, multilingual interfaces, mobile access, and practical training to improve engagement and compliance. |
Protocol amendments | Choose a configurable RTSM that allows safe sandbox testing and quick deployment of mid-study updates without disruptions. |
The Role of AI and Automation in the Future of RTSM
Automation and artificial intelligence are redefining how clinical trials are managed. Within RTSM systems, these technologies go beyond task automation to enable intelligent, data-driven decision-making.
- Automated Workflows: Modern RTSM platforms use automation to streamline repetitive processes such as patient randomization, kit assignment, and supply resupply. This reduces manual effort, minimizes human error, and accelerates overall study timelines.
- AI-Driven Forecasting: Artificial intelligence enhances supply forecasting by analyzing real-time enrollment data, dosing patterns, and site performance. Predictive algorithms can anticipate shortages or overages before they occur, improving efficiency and reducing waste.
- Intelligent Decision Support: Machine learning models can detect trends such as delayed shipments, patient dropouts, or data inconsistencies, and alert study teams to take corrective actions proactively.
- Enhanced Compliance and Quality: Automation ensures consistency in audit trails, validation checks, and reporting, while AI-driven anomaly detection strengthens data accuracy and regulatory readiness.
As these technologies mature, RTSM will evolve from being a trial management tool into an intelligent control center, continuously learning from data to optimize randomization, supply, and operational strategies in real time.
Conclusion
As technology continues to evolve, RTSM will play an even greater role in how trials are designed and executed. Automation and AI are making it possible to manage complexity with precision - anticipating needs, reducing manual effort, and improving data confidence. The result is a more connected, responsive trial environment where operations run smoothly, and insights are available in real time.
Clinion RTSM
Clinion RTSM redefines randomization and supply with the industry’s best integration. Automate patient randomization, kit assignment, reassignment, and returns through Clinion EDC without logging into multiple systems. Deploy in days, reduce costs, and ensure accuracy and compliance. Designed for flexibility, Clinion RTSM supports studies ranging from the very simple to the highly complex.
External References

A marketing leader with a sharp focus on strategic clarity, positioning, and GTM alignment. At Clinion, he drives marketing initiatives that connect narrative precision with measurable growth, ensuring the company’s AI-powered innovations resonate deeply across the life sciences industry.
FAQS
Frequently Asked Questions
RTSM platforms can accommodate approved mid-study changes, such as adjusting randomization parameters, adding new sites or cohorts, or updating visit schedules. They also integrate with tools like eConsent and remote monitoring systems, enabling decentralized and hybrid trial operations with centralized oversight.
By automating randomization and supply processes, RTSM minimizes human error and ensures the correct treatment is dispensed to the right participant. Built-in audit trails and validation checks maintain full traceability, protecting data integrity and patient safety.
Yes. Modern RTSM systems are designed to support multi-site and multi-country studies, managing varying regulations, time zones, and shipment logistics. They can also centralize data from multiple protocols under one platform for consistent oversight.
Validation follows GxP and 21 CFR Part 11 requirements. It includes installation, operational, and performance qualification (IQ, OQ, PQ) testing to confirm that the system performs as intended, maintains data integrity, and complies with all regulatory standards.
Most vendors provide role-based training for sponsors, sites, and CRAs. This typically includes virtual sessions, sandbox testing, and quick-reference guides to ensure users can navigate the system confidently before the study goes live.
A robust RTSM can easily scale from small Phase I studies to large, global Phase III trials. Its modular architecture allows new sites, treatment arms, or protocols to be added without rebuilding or interrupting ongoing operations.
RTSM systems can connect directly with central depots and third-party logistics providers to track shipments, monitor temperature excursions, and manage inventory transfers. This ensures real-time visibility and compliance across the full supply chain.
Clinion RTSM combines randomization and supply management with deep integration into Clinion EDC. It automates critical workflows, supports adaptive studies, and can be deployed in days, offering unmatched flexibility and cost efficiency.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


