CTMS
CTMS for Sponsors and CROs
Gain complete visibility and centralized control over your trials with our clinical trials management system (CTMS).
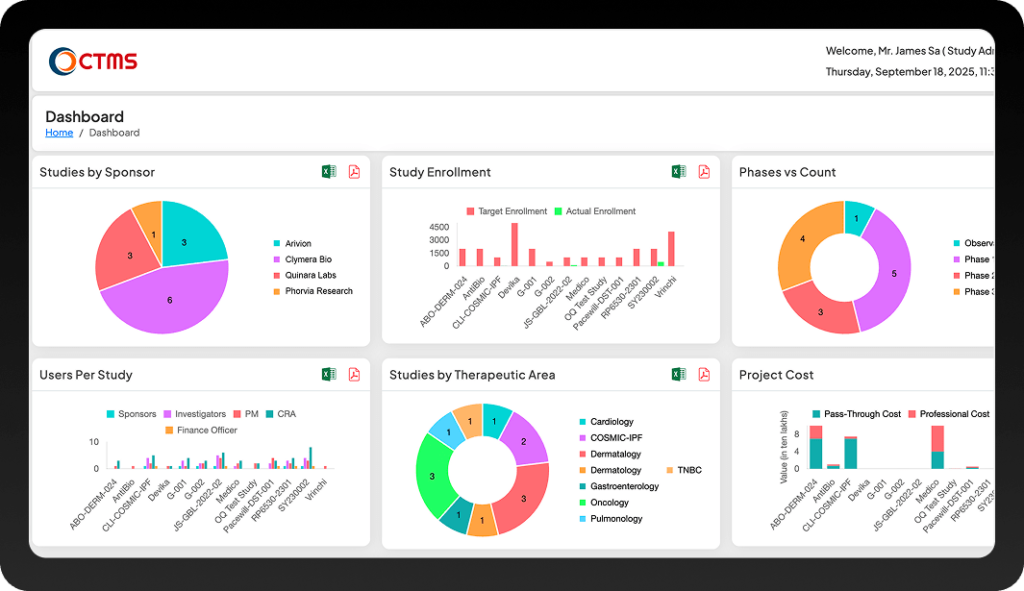
Why CTMS ?
Join 100+ Customers Accelerating Trials Across Phase I to IV
Countries
Subjects
Customers
Clinical Trials
Biotech Studies
FDA Studies
Deep Integration
Our CTSM system is built to be the central hub of your eClinical ecosystem. It connects natively with Clinion’s EDC, RTSM, and eTMF, and offers flexible integration with your existing third-party systems.
Simple to Setup
Get your studies up and running faster. With the simplicity of our setup process, we ensure an extremely smooth onboarding experience.
Easy to Use
Designed with the end-user in mind, Clinion CTMS software features an intuitive interface that reduces training time and increases adoption.
Features
Key Features of Clinical Trial Management Software (CTMS)
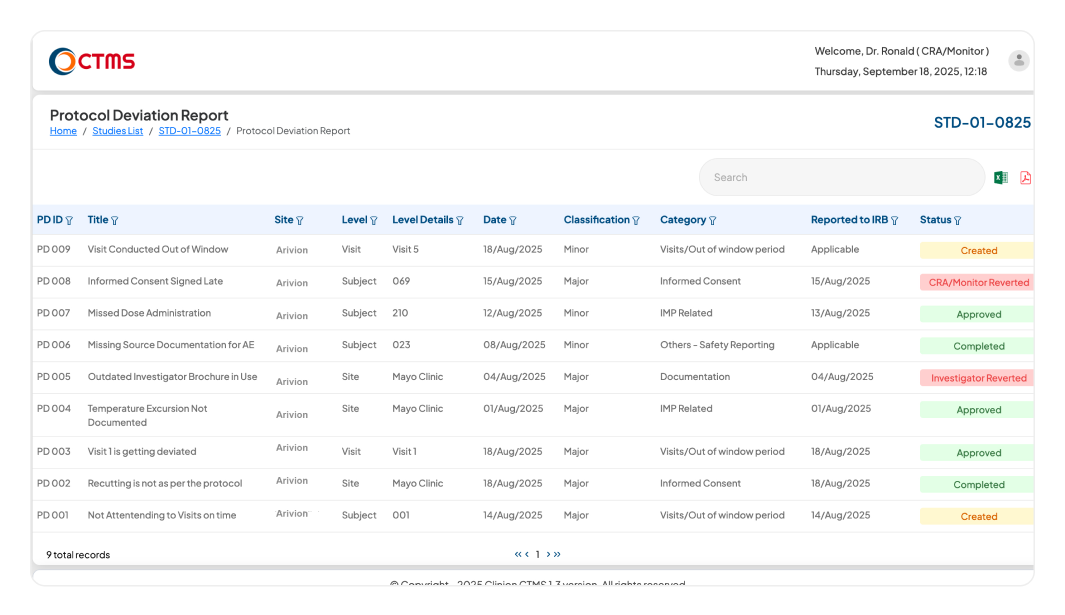
PROTOCOL DEVIATION
Proactively Manage Deviations and Protect Trial Integrity
Systematically identify, document, and manage all protocol deviations in a centralized log.
Monitor all deviations from a central dashboard, using powerful filters by PD ID, title, date, classification, or category. Automated email notifications for key events, including submission, review, and sign-off, ensure the entire team (CRC, CRA, PM, and Investigator) stays informed and aligned at every step.
Monitor all deviations from a central dashboard, using powerful filters by PD ID, title, date, classification, or category. Automated email notifications for key events, including submission, review, and sign-off, ensure the entire team (CRC, CRA, PM, and Investigator) stays informed and aligned at every step.
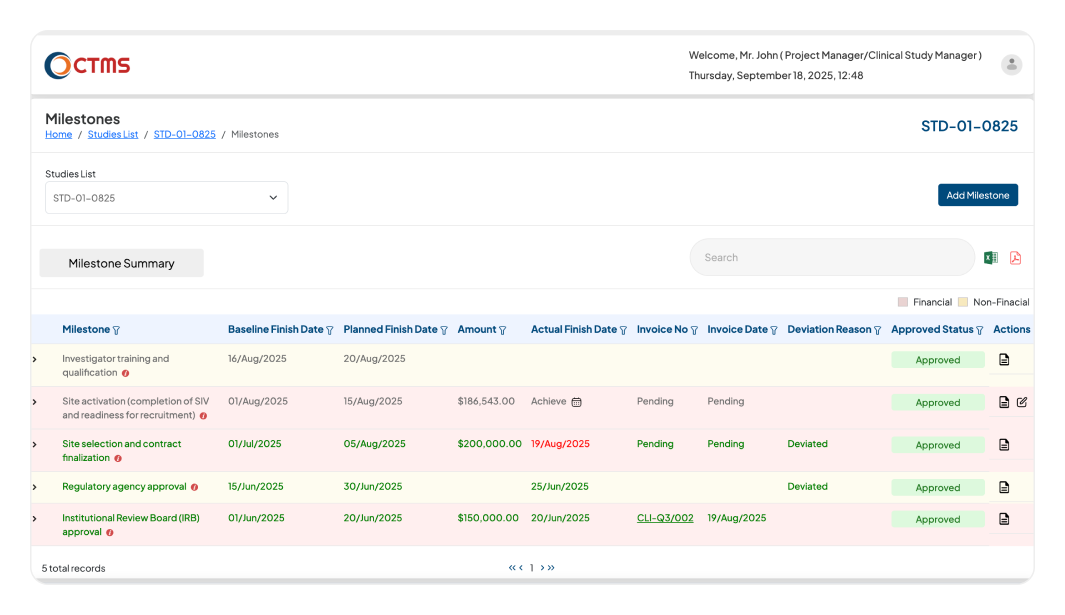
STUDY MILESTONE
Ensure your Clinical Trials are Always Delivered on Time
Gain a complete, end-to-end view of your entire study timeline, from the trial initiation to final database lock with our clinical trials management system (CTMS)
Instantly compare your planned timelines with actual progress in real-time. This provides an immediate overview of your study's status, so you always know if you're on schedule.
Identify and flag any deviations from your study plan.
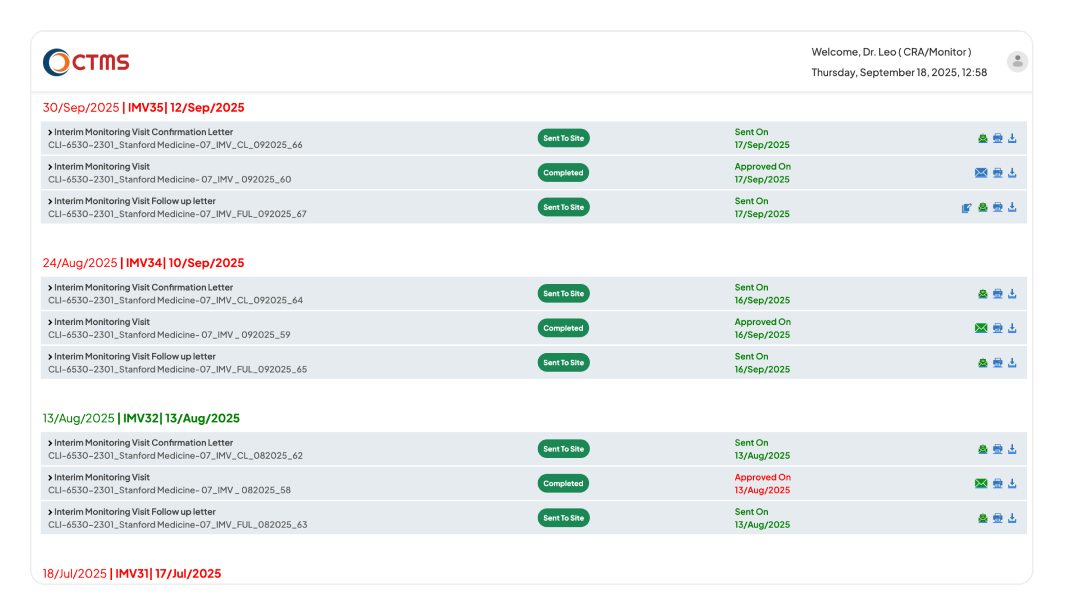
REPORTS & LETTERS
Accelerate Compliant Reporting with Workflows
Enforce timely reviews and approvals to ensure accuracy, consistency, and full compliance across all sites.
Gain complete control over the entire report and letter lifecycle. You can start reports and letters faster using the templates tailored to customers, and attach supporting documents for full context. Automate review and approval with configurable workflows (L1 to L5), while tracking all related action items to closure so nothing is missed. Instantly monitor the status of all correspondence on real-time dashboards and maintain a complete, time-stamped audit trail for constant inspection readiness.
Bring structure and accountability to your post-visit reporting with a clear, configured workflow. After a visit, the system prompts the CRA/PM-CSM to create all necessary documentation - the Confirmation Letter, Thank You Letter, Non-Selection of Investigational Sites Letter, Activation Letter, Follow-up Letter, and Monitoring Report, within a mandatory timeline. The report is then automatically routed to the L2 level, who has complete flexibility to approve it, add comments, or revert it for updates.
Never lose track of important site tasks with our simple tracking system. CRAs can quickly add action items during visits, set priorities and due dates, and assign them to the right people. Pending tasks automatically move forward to future reports until they're completed. You can close tasks during on-site or remote visits, and every item gets a unique ID for easy tracking. Real-time dashboards show you the status of all tasks across all sites, so you always know what needs attention.
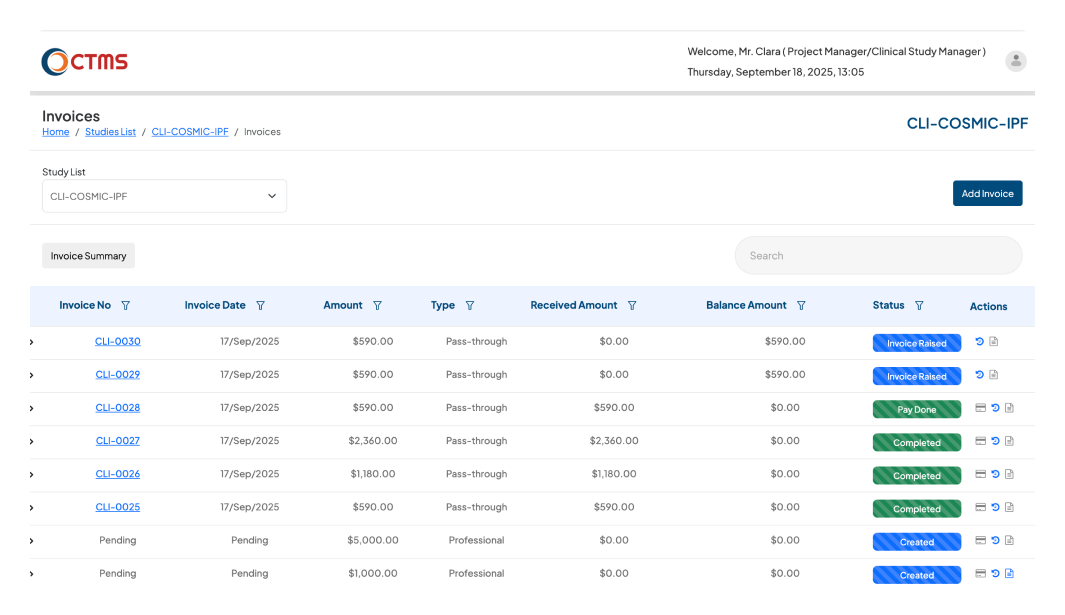
FINANCIAL MANAGEMENT
Simplify Invoices, Expenses & Payments
With Clinion’s CTMS software, you eliminate manual spreadsheets, reduce delays, and gain end-to-end visibility into your trial’s financial health.
Once the milestone is marked as achieved, the CTMS will automatically trigger an email and allow the Project Manager to raise invoices for achieved milestones from professional costs.
Automate your entire expense lifecycle, from submission to payment, with a powerful and configurable workflow. Empower your study personnel (like PIs and CRCs) and external Vendors to submit Site and Vendor expenses directly into the system. Each claim is then automatically routed through a configurable approval hierarchy. Approvers at any level can review and take action. If a claim is reverted, it's instantly returned to the submitter to be corrected and resubmitted. Once an expense passes all approval stages, it is cleared for final payment by finance, ensuring full visibility and compliance.
Ensure constant inspection readiness with a complete, immutable, and time-stamped financial history. Every invoice, approval, and payment is logged automatically for full compliance.
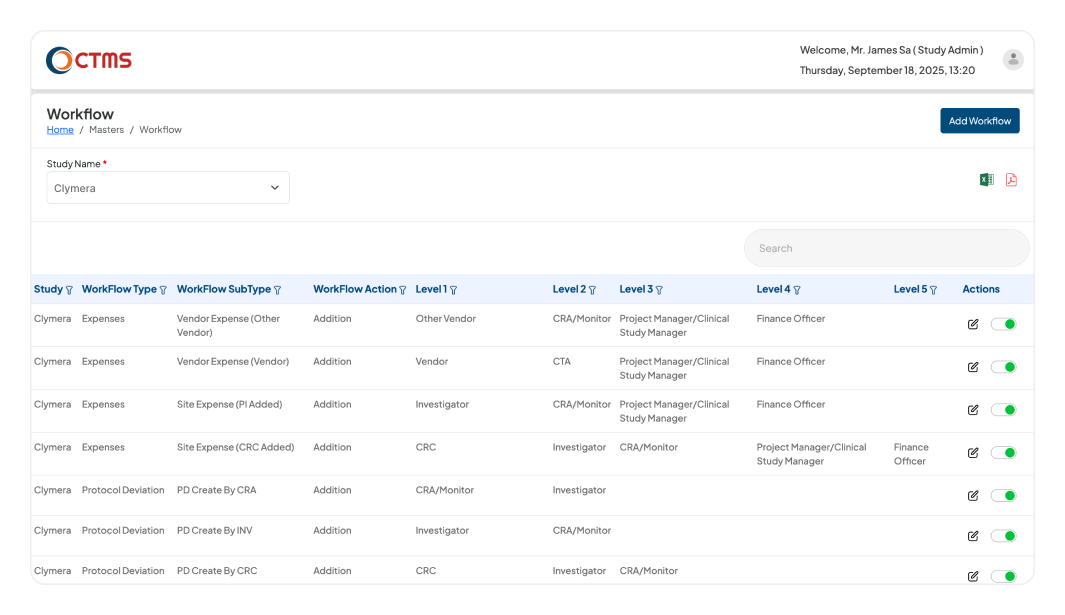
SYSTEM ADMINISTRATION & CONFIGURATION
Configure Your CTMS Software to Match Your Processes
Empowers Study Admin to define all master data, financial rules, and multi-level approval workflows, ensuring the CTMS system operates exactly as per your organization processes.
Define your organizational chain of command within the CTMS software. The Study Admin can build a five-level approval hierarchy for key processes like Milestones, Reports & Letters, Protocol Deviations, Expenses (Site & Vendor), and Monitoring Plans. Actions are automatically routed for approval based on role level. For example, a level-one user's action can require approval from both level two and level three.
Keep your entire team informed and on track by configuring 100+ automated notifications and reminders for critical trial events. The Study Admin has full control to modify notification details, set event triggers (like status updates, task completions, or system alerts), and define the precise timing and frequency for all reminders. Whether it’s an upcoming site visit, overdue action item, pending report approval, or payment milestone, our CTMS system ensures the right people are alerted at the right time.
Add, manage, and edit the details for all key partners, including Sponsors and Vendors. Configure the Tax details and the Invoice formatting of the organization for invoicing.
Configure your workspace's core operational settings in one central place. Update your Company Profile with your organization's logo and banking details. Manage site geographies by adding cities and viewing state lists.
Set the Study Calendar by defining weekends and adding company holidays, either manually or via file upload, to ensure accurate project scheduling.
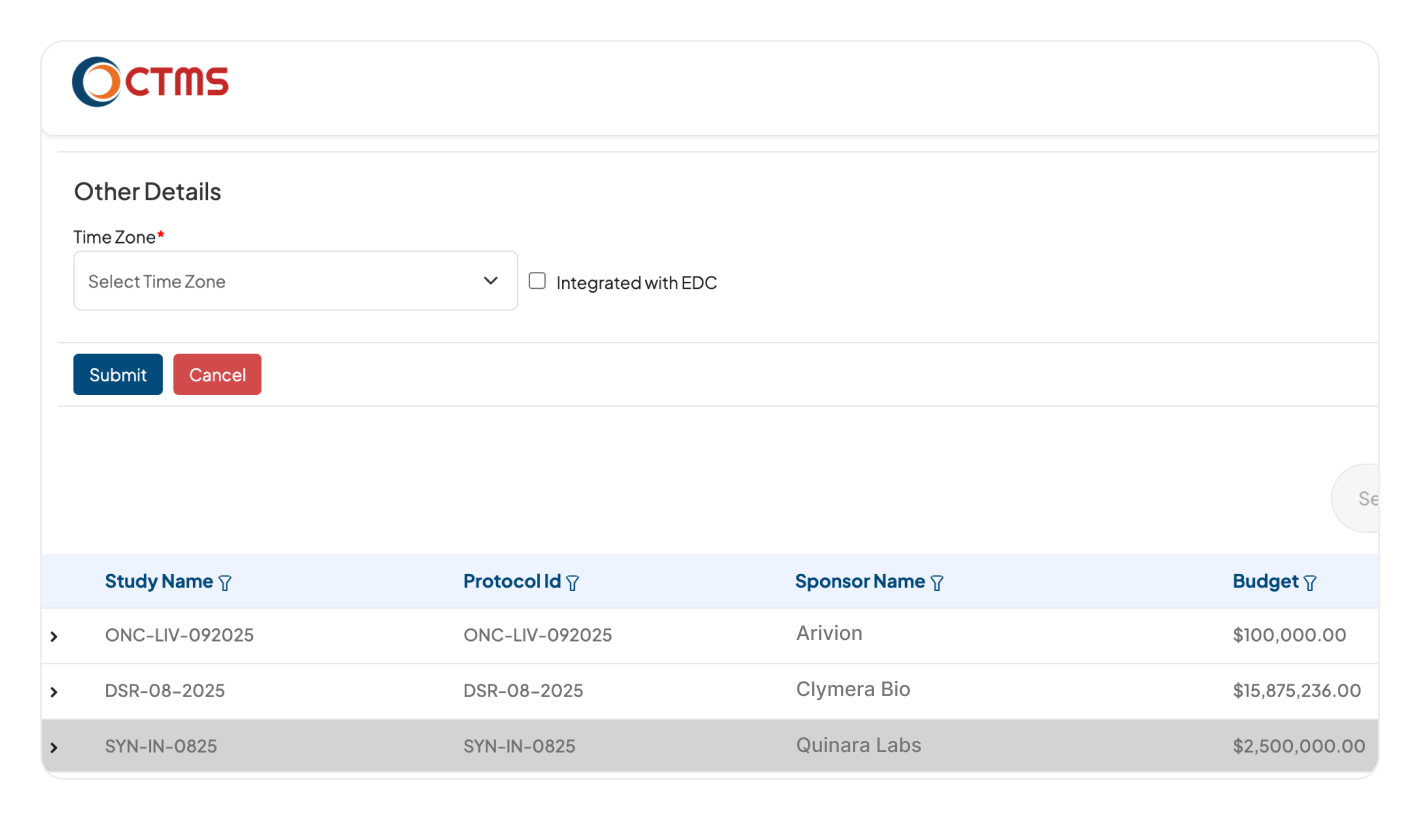
INTEGRATION
Connect Every System for Complete Trial Oversight
Our CTMS system connects seamlessly with the Clinion suite (EDC, RTSM, eTMF). All clinical, operational, and regulatory data flows into one unified platform for real-time insights.
Break down data silos across your entire tech stack. Connect with your existing third-party EDC, RTSM & eTMF systems, ensuring that all relevant trial data flows into one central, unified platform.
FAQS
Frequently Asked Questions
Discover quick solutions to your Clinion CTMS system queries
Sponsors, CROs, study managers, and site coordinators managing multi-site trials, timelines, or budgets all benefit from a CTMS. Any clinical operations team looking to improve visibility, efficiency, and control can gain from using a CTMS.
CTMS implementation involves assessing operational needs, choosing the right vendor, migrating data, configuring the system, training users, validating workflows, and rolling out in phases to ensure smooth adoption.
A CTMS improves team collaboration through shared dashboards, task tracking, milestone visibility, and real-time communication. It helps prevent delays, reduce miscommunication, and align teams across functions and geographies.
Clinion CTMS goes beyond tracking, it actively drives trial execution. It centralizes milestones, budgets, and site activities on a single platform, enabling faster decisions and tighter coordination. Designed for clarity and speed, it helps teams stay compliant and on track across the study lifecycle.
By automating workflows and providing real-time financial dashboards and inventory alerts, Clinion CTMS cuts operational costs by up to 30%. It eliminates manual steps and reduces redundancies, freeing teams to focus on high-value work.
Clinion CTMS accelerates study startup by 50% through pre-built workflows, centralized task management, and easy CRA assignment. It streamlines protocol setup and milestone planning, helping teams launch and manage trials with greater speed and accuracy.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Responsible AI
Clinion follows Responsible AI principles, ensuring its AI tools are built for safety and reliability, and remains committed to Data Privacy and Security at every step.
- Accountability
- Transparency
- Privacy & Security
- Reliability & Safety
- Fairness
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


