Insights / Blog / Clinical Trials & AI
How Multi-Agentic AI Is Revolutionizing Clinical Trials
- Abriti Rai
- December 26, 2025

On this Page
- Summary
- The AI Evolution Curve: From Analytical to Multi-Agentic
- How Multi-Agentic AI Is Transforming Clinical Trial Processes
- Why Multi-Agentic AI Matters: The Tangible Benefits
- Challenges Associated with Multi‑Agentic AI in Clinical Trials
- The Future is Here
- External References
- Summary
- The AI Evolution Curve: From Analytical to Multi-Agentic
- How Multi-Agentic AI Is Transforming Clinical Trial Processes
- Why Multi-Agentic AI Matters: The Tangible Benefits
- Challenges Associated with Multi‑Agentic AI in Clinical Trials
- The Future is Here
- External References
Summary
Multi-Agentic AI in clinical trials is a coordinated network of specialized AI agents that think, decide, and adapt together. Acting like a self-orchestrating research team, they accelerate study design, monitoring, and reporting while boosting accuracy and compliance.’
Every industry is experiencing an AI revolution. Yet, most conversations still focus on single, siloed AI models that solve one problem at a time.
Clinical trials, however, are far too complex for such narrow solutions. They span hundreds of interconnected processes, thousands of data points, and constant decision-making under strict regulatory oversight. We are now entering a new era: one where AI is not just a passive tool, but an active, thinking collaborator.
This article explores Multi-Agentic AI, where multiple intelligent agents work together, share knowledge, and adapt dynamically throughout the clinical trial lifecycle. It’s not just about faster execution; it’s about coordinated intelligence.
The AI Evolution Curve: From Analytical to Multi-Agentic
Traditional AI in clinical trials has primarily been used to automate narrow tasks. These systems work on predefined rules or static models. While helpful, they are limited, rigid under changing conditions, and heavily reliant on human oversight.
What’s emerging now is a shift from automation to agency. Intelligent systems are being designed not just to execute instructions, but to understand goals, make decisions, and adapt in real time. This marks the progression from Single-Agent AI to Agentic AI, and further toward Multi-Agentic AI.
Single-Agent AI | Agentic AI | Multi-Agentic AI |
|
|
|
How Multi-Agentic AI Is Transforming Clinical Trial Processes
Clinical trials are highly interconnected systems, where each stage feeds into the next, and a setback in one can derail the entire process.
Multi-Agentic AI brings together a team of specialized agents under a central orchestrator. They collaborate, cross-check, and adapt in real time, transforming fragmented workflows into a seamless, intelligent operation that keeps trials moving efficiently from start to finish.
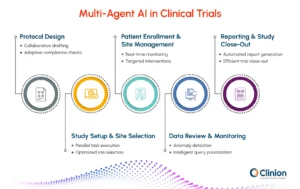
Let’s look at how a multi-agentic system enhances each stage of a clinical trial:
Protocol Design and Authoring
In protocol development, Multi-Agentic AI coordinates a network of autonomous yet collaborative capabilities working toward the shared goal of producing a high-quality, compliant study plan.
While one capability independently drafts the initial protocol using trial objectives, therapeutic area expertise, and prior data, others assess operational feasibility, ensure logical consistency, and verify alignment with ICH-GCP and applicable regulations.
Agents exchange findings and reconcile differing perspectives instantly, updating the draft as new insights, constraints, or regulatory changes arise.
Study Setup and Site Selection
During study setup, Multi-Agentic AI enables activities to run in parallel instead of sequentially. Autonomous modules initiate tasks and manage dependencies without waiting for human prompts.
Agents propose optimized CRF templates, patient assignment schemes, and drug supply plans, while predictive site analysis evaluates historical performance and recruitment potential to identify optimal locations. As new operational or logistical data emerges, agents adjust plans collectively to present the most viable setup.
Patient Enrollment and Site Management
Once recruitment begins, Multi-Agentic AI monitors enrollment patterns continuously and triggers targeted outreach when early-warning indicators appear. It evaluates site performance, patient retention, and emerging risks, coordinating with resource allocation or outreach agents when interventions are needed.
These strategies are informed by patterns from previous trials, enabling the system to choose the most effective approaches under similar conditions. The feedback loop helps maintain momentum, prevents bottlenecks, and reduces delays.
Data Review and Monitoring
In the data management phase, Multi-Agentic AI applies ongoing, self-directed quality oversight. It scans live data streams for irregularities, safety signals, and operational risks, prioritizing issues that warrant human review.
Query generation, tracking, and resolution are handled in coordination with targeted source-data verification, focusing effort where it has the most impact. Insights are shared instantly, so anomaly detection informs query prioritization, and verification results feed back into monitoring algorithms.
The system refines its detection criteria continuously based on previous trial data, improving both speed and accuracy.
Reporting and Study Close-Out
At study completion, Multi-Agentic AI synchronizes report generation, statistical analysis, and compliance checks as interconnected processes. Analytical results, tables, listings, and figures are validated and cross-referenced with regulatory standards.
A compliance agent autonomously flags and resolves inconsistencies before finalization. Agents adjust sequencing and focus based on data readiness, drawing on lessons from past close-outs to streamline the process. This proactive coordination minimizes delays and ensures a smooth, well-governed trial closure.
Why Multi-Agentic AI Matters: The Tangible Benefits
Unlike traditional AI models that operate in isolation on individual tasks, Multi‑Agentic AI brings multiple intelligent agents together to collaborate, communicate, negotiate, and adapt as a unified system. This coordinated approach delivers benefits that go far beyond simple automation.
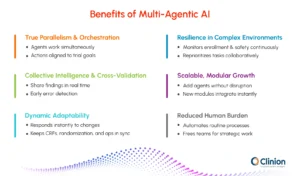
True Parallelism & Orchestration
- Agents work simultaneously across multiple trial functions
- Orchestration layer aligns actions with overall trial objectives
Collective Intelligence & Cross-Validation
- Agents share findings and verify outputs in real time
- Early error detection reduces false positives and improves data integrity
Dynamic Adaptability
- Instantly to trial changes like protocol amendments
- Linked updates keep CRFs, randomization, and operations in sync
Resilience in Complex Environments
- Monitors enrollment, regulations, and safety signals continuously
- Reprioritizes tasks collaboratively to maintain compliance and progress
Scalable, Modular Growth
- Add new agents without disrupting workflows
- New modules integrate instantly into decision-making
Reduced Human Burden
- Handles routine, coordinated processes autonomously
- Frees clinical teams for higher-value strategic work
Real-World Application: Clinion’s Multi-Agentic AI for Data Review
Clinion’s Multi-Agentic AI brings together multiple intelligent agents to autonomously interpret protocols, generate datasets, identify discrepancies, and manage queries, without manual prompting. This collaborative, agent-based approach removes repetitive tasks and allows data managers to focus on higher-value decisions.

Results:
4X faster data review cycles - AI accelerates the review of clinical trial data, completing cycles up to four times quicker than traditional methods.
85% of queries auto-generated - Most data queries are automatically created by the system, leaving only review and approval for the team.
Up to 60% reduction in data manager effort - Automated processes significantly cut manual workload, allowing data managers to focus on critical oversight and analysis.
This innovative approach is reshaping how clinical data management teams operate, driving greater efficiency and accuracy across trials.
Challenges Associated with Multi‑Agentic AI in Clinical Trials
While Multi-Agentic AI holds immense transformative potential, successfully integrating these sophisticated systems into clinical trials presents unique and complex challenges.
Regulatory Trust & Transparency
Multi-agent systems must document how each agent made decisions, collaborated, and resolved conflicts. Clear audit trails, validated outputs, and interpretable explanations are essential to meet evolving FDA, EMA, and ICH-GCP requirements.
Data Quality & Integration
These systems rely on high-quality, interoperable data. Real-time harmonization across diverse sources and alignment to standards like CDISC or HL7 FHIR is critical to ensure agents receive clean, structured inputs.
Human–AI Collaboration & Change Management
Trust and adoption depend on clear governance, human-in-the-loop oversight, and transparency in recommendations. Ongoing training helps teams know when to trust AI output and when human judgment is required.
Scalability & Customization
While the architecture is scalable, tailoring agents to specific protocols or therapeutic areas demands significant domain expertise. Transfer learning can speed setup, but rigorous validation is required before deployment.
The Future is Here
Agentic AI, with its capacity for autonomous decision-making and advanced problem-solving, is already transforming the clinical trial landscape. While today’s applications show significant promise, we are only beginning to unlock the full potential of these systems.
Future Multi-Agentic AI platforms will seamlessly navigate the complexities of trial design, execution, and management, delivering unprecedented precision and agility. Integrating Agentic AI with emerging technologies such as quantum computing and advanced robotics will push the boundaries even further. This synergy will enable rapid data analysis, real-time monitoring, and predictive insights that were once unimaginable.
These advancements will be essential for meeting evolving regulatory demands while maximizing operational efficiency. The shift is clear: clinical trials are moving beyond isolated tools toward coordinated, goal-driven collaboration among intelligent agents.
The future of clinical research lies in self-orchestrating, adaptive AI networks powered by systems that think and act together to redefine the future of clinical research.
External References

Abriti Rai writes on the intersection of AI, automation, and clinical research. At Clinion, she develops content that simplifies complex innovations and highlights how technology is shaping the next generation of data-driven clinical trials.
FAQS
Frequently Asked Questions
Multi-Agentic AI in clinical trials is a coordinated system where multiple specialized AI agents manage different aspects of the trial process. Working under a central orchestration layer, these agents share data, align decisions, and adapt in real time. This enables end-to-end clinical trial automation, covering protocol design, site selection optimization, patient recruitment, data monitoring, and study close-out, making trials faster and more efficient.
Most traditional AI tools focus on a single task and require frequent human oversight. Multi-Agentic AI connects several autonomous agents that understand trial objectives, work in parallel, and make independent decisions. This integrated approach links functions like protocol compliance, site management, and real-time trial monitoring, creating a more adaptive and connected clinical trial process.
Multi-Agentic AI enables trial processes to move forward simultaneously, with agents continuously sharing insights and refining each other’s work. They learn from outcomes, spot issues earlier, and adjust instantly to changes, turning what is normally a slow, linear process into a fast, interconnected, and highly resilient system.
No, it doesn't. However, it handles far more complex and interconnected tasks than standard AI, and removes much of the operational burden. Human expertise remains central for oversight, decision-making, and ensuring ethical and scientific integrity.
Yes. The modular architecture of Multi-Agentic AI allows sponsors to customize agents for specific therapeutic areas, trial phases, or regulatory environments. It can be configured to integrate with existing clinical trial management systems (CTMS), support local compliance requirements, and optimize processes for the unique demands of each study.
Multi-Agentic AI automatically adjusts when new conditions arise, such as protocol amendments, recruitment challenges, or updated regulatory requirements. For example, a protocol change can instantly trigger updates to case report forms (CRFs), patient randomization plans, and supply chain schedules, while keeping all AI agents aligned for continuous compliance and real-time trial monitoring.
Common challenges include ensuring data quality, meeting strict regulatory compliance, integrating with existing platforms, and building trust among clinical teams. Overcoming these requires robust validation, standardized data formats, clear governance frameworks, and ongoing collaboration between human teams and AI agents.
Yes. Companies like Clinion are already using Multi-Agentic AI for data review automation in live trials. Their coordinated AI agents interpret protocols, detect discrepancies, and manage queries autonomously, making data review up to 4x faster and reducing data manager workloads by as much as 60%. This accelerates trial timelines, enhances data accuracy, and demonstrates the real-world value of AI-powered clinical trial automation.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


