ePRO isn’t just digitized data collection. It’s real-time, structured, regulatory-grade intelligence, designed to enhance compliance, cut down queries, and keep patients engaged.
Despite operating in a digitally driven world, many clinical trials continue to rely on old-fashioned paper diaries to collect patient-reported data. As recently as 2018, nearly half of all studies capturing patient outcomes were still using paper. And that dependence hasn’t vanished overnight.
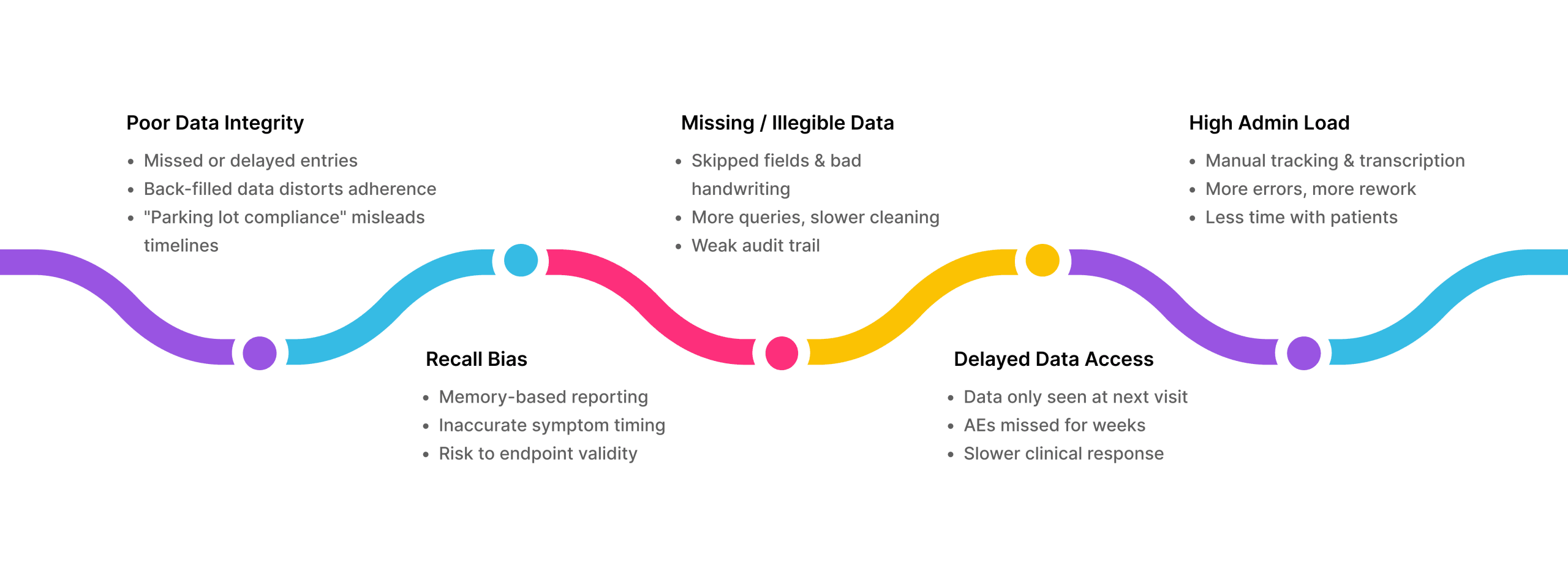
Paper diaries come with known risks: missing data, unreadable handwriting, and “parking lot compliance”, when patients rush to fill in days of entries just before a site visit. These issues compromise data integrity, delay insights, and strain already tight timelines.
This guide unpacks why ePRO (electronic Patient-Reported Outcomes) isn’t just about digitizing forms. It’s about transforming how clinical trials capture, monitor, and act on patient-reported data, unlocking better compliance, cleaner data, and real-time oversight that paper can never deliver.
Why ePRO Is Reshaping Patient Data Capture
ePRO systems use digital platforms such as mobile apps, web portals, and tablets to capture patient health data directly, without clinician interpretation. Compared to paper, ePRO ensures:
- Real-time data capture
- Stronger compliance
- Cleaner, more complete data
- Reduced site burden
It’s not just digital convenience. It represents a fundamental shift in how trials collect, monitor, and act on patient feedback and brings speed, structure, and intelligence to a process long overdue for transformation.
The Problems with Paper Diaries
ePRO: A Step-Change in Accuracy, Oversight, and Experience
Given the well-documented drawbacks of paper diaries, more sponsors and CROs are adopting ePRO systems. ePRO addresses the limitations of paper while offering significant advantages across accuracy, compliance, oversight, and sustainability.
Improved Data Accuracy & Integrity
- Real-time entries, time-stamped automatically
- No back-filling, no fake compliance
- Configured forms require completion and prevent conflicts
Real-Time Access & Oversight
- Entries visible instantly to sites and monitors
- AEs detected and escalated earlier
- Automated alerts if patients skip inputs
- Enables mid-study decisions and intervention
Higher Patient Engagement & Compliance
- BYOD support makes it user-friendly
- Intuitive, mobile-first design
- Automated reminders reduce drop-offs
- Industry surveys show 75%+ patient preference for ePRO
Regulatory Confidence & Traceability
- 21 CFR Part 11 compliant
- Every data point time-stamped and user-linked
- Audit-ready by default
- Endorsed by FDA and EMA for use in primary endpoints
Eco-Friendly & Operationally Efficient
- Eliminates printing, shipping, and storage
- Reduces manual handling and transcription
- Supports sustainable, modern trial ops
Why ePRO Isn’t Just a Digital Replica
It’s easy to assume that ePRO simply mimics a paper diary on a screen, but that’s a significant underestimation. In reality, ePRO isn’t just digitization. Modern ePRO solutions go beyond basic form-fill functions to actively enhance the way patient-reported data is collected, managed, and interpreted. Here’s how:
Dynamic, Adaptive Questioning
- Logic-driven flows skip irrelevant questions
- Avoids contradictory inputs
- Personalized based on patient responses
Seamless Integration & BYOD
Richer Data, Not Just More Data
- Capture of metadata (timestamp, geo, device)
- Images, video uploads (where applicable)
- Enables real-world evidence capture
Real-Time Intelligence for Sponsors
- Immediate analytics on adherence, symptoms, and safety
- Allows adaptive interventions mid-study
- No waiting for site visits to understand patient experience
From Paper to Progress: ePRO Is the Path Forward
Still using paper? You’re accepting slower timelines, questionable data, and avoidable admin load.
Today’s ePRO platforms are:
- User-friendly and intuitive for all age groups
- Built to integrate seamlessly with existing clinical systems
- Designed for scalability across geographies and trial sizes
If you’re still weighing the switch, consider this: paper may feel comfortable, but it doesn’t match the demands of today’s fast-moving, patient-centric trials. ePRO doesn’t just replace paper, it elevates everything that comes after it.
With ePRO, the impact is measurable:
- Up to 50% fewer queries
- Improved retention and protocol adherence
- Faster access to clean, actionable data
What to Ask When Choosing an ePRO Platform
- Does it support BYOD and multilingual deployment?
- How does it handle real-time alerts and patient engagement?
- Can it integrate with EDC, CTMS, and wearables?
- Is the audit trail 21 CFR Part 11 validated and traceable?
- Can it adapt to both decentralized and hybrid trial models?
These aren’t nice-to-haves, they’re the new baseline.
Clinion ePRO: Data That Starts Clean and Stays Clean
Clinion ePRO goes beyond simple digitization – it drives meaningful outcomes. Empowering patients to report outcomes with ease and accuracy, anytime and anywhere.
- 89% retention improvement
- Trusted by 20,000+ patients across global trials
- Fully compliant, multilingual, and BYOD-ready
- Instant access, traceable entries, and effortless integration with your data ecosystem
“ We cut query volume by 50% and accelerated database lock by 12 days using Clinion ePRO.
Frequently Asked Questions (FAQ)
What is the difference between ePRO and eCOA?
eCOA (electronic Clinical Outcome Assessment) is a broad term that includes all digitally captured clinical outcome data, whether reported by patients (ePRO), clinicians (eClinRO), observers like caregivers (eObsRO), or through performance-based tests (ePerfO). ePRO is a specific subset of eCOA that focuses only on data reported directly by patients. Both eCOA and ePRO improve data quality, reduce manual errors, and support greater patient engagement.
What are the benefits of ePRO in clinical trials?
ePRO improves data accuracy, completeness, and timeliness through real-time, direct patient input. It reduces manual errors, underreporting, and administrative burden with automated checks and prompts. Remote access and user-friendly design enhance patient compliance and retention. Faster insights support better safety monitoring and trial efficiency.
What are the limitations of paper diaries in clinical trials?
Paper diaries are prone to transcription errors, illegible entries, and missing data, which can compromise accuracy and delay analysis. Participants often fill them out retrospectively, leading to recall bias and unreliable data. Paper lacks time-stamping, making it difficult to verify when entries were made. Managing and storing physical documents is also labor-intensive, space-consuming, and poses risks to data security and confidentiality.
Can Clinion ePRO integrate with other platforms or operate as a standalone platform?
Yes, Clinion ePRO offers flexible deployment. It seamlessly integrates with EDC and other clinical systems for unified workflows but can also operate as a fully functional standalone platform, ensuring adaptability to diverse trial needs.
What kind of information does ePRO gather from patients?
ePRO systems collect subjective health data that only patients can accurately report. It captures personal experiences such as symptom intensity, emotional well-being, physical functioning, and how treatments affect their daily lives. This qualitative insight adds essential context to clinical endpoints and helps researchers better understand the patient journey over time.
Is Clinion’s ePRO data collection secure and compliant?
Yes, Clinion’s ePRO solutions are built with robust security measures and strict regulatory compliance in mind. They use advanced encryption, secure data transmission protocols, and comprehensive audit trails to protect patient privacy and ensure data integrity. Clinion’s ePRO functionality is fully compliant with GDPR and adheres to other regulations like 21 CFR Part 11 and ISO 2001, ensuring all digital data capture is secure and trustworthy for clinical trials.
What sets Clinion ePRO apart from other ePRO solutions?
Clinion ePRO stands out with its intuitive interface, real-time data capture, and seamless EDC integration, while also functioning as a standalone solution. Unlike standard tools, it offers robust audit trails, automated reminders, and multilingual support, ensuring regulatory compliance and global scalability. Clinion ePRO simplifies patient reporting and enables faster, data-driven decisions, making it a smarter end-to-end solution for modern clinical trials.
Can ePRO solutions be tailored for specific studies?
Yes. Modern ePRO solutions are highly adaptable and customizable to meet the unique needs of different clinical trials. They can be configured to match specific study protocols, collect diverse outcome measures, and adapt to various therapeutic areas and patient populations. This flexibility ensures that the digital data capture method aligns perfectly with the study's design, optimizing data relevance and patient engagement.
What is the future outlook for ePRO in clinical trials?
The future of ePRO is set to revolutionize patient-reported outcomes. It will become the industry standard, providing richer, real-time insights by seamlessly merging with wearables, AI, and other digital health technologies. This advanced integration will enable earlier safety interventions, significantly enhance patient participation through user-friendly Bring Your Own Device (BYOD) options, and streamline the shift toward decentralized clinical trials. Delivering reliable, secure, and easily accessible data will remain paramount to fast-track new therapies and improve patient care worldwide.



