Insights / Blog / eConsent
eConsent in Clinical Trials: A Comprehensive Guide to Electronic Informed Consent
- Abriti Rai
- January 12, 2026

On this Page
- Summary
- What is eConsent in Clinical Trials?
- Why eConsent Matters Today
- How eConsent Improves Participant Experience
- How eConsent Supports Enrollment and Retention
- Operational Advantages for Study Teams
- Paper vs eConsent: A Quick Comparison
- Common Misconceptions About eConsent
- Conclusion
- Summary
- What is eConsent in Clinical Trials?
- Why eConsent Matters Today
- How eConsent Improves Participant Experience
- How eConsent Supports Enrollment and Retention
- Operational Advantages for Study Teams
- Paper vs eConsent: A Quick Comparison
- Common Misconceptions About eConsent
- Conclusion
Summary
Electronic informed consent (eConsent) in clinical trials has moved from a supporting role to a central part of patient engagement. Modern eConsent platforms help participants understand study expectations with greater clarity and give sites a structured way to manage consent data with higher accuracy.
What is eConsent in Clinical Trials?
Electronic informed consent, or eConsent, refers to the digital process of providing study information and obtaining participant consent. Instead of relying on long paper forms, eConsent uses multimedia and interactive formats that help participants understand the study more clearly.
eConsent forms are now common across clinical research because they improve comprehension, support transparency, and ensure that every version of the consent is tracked and stored correctly.
Why eConsent Matters Today
Traditional consent processes often create barriers. Participants may find lengthy documents difficult to interpret, and sites may struggle to manage version control across multiple locations.
eConsent addresses these issues by improving:
Participant understanding
Data accuracy
Oversight for sites and sponsors
Access for global participants
A smoother process benefits everyone involved in the study.
How eConsent Improves Participant Experience
Participants are more confident and informed when study information is presented clearly and accessibly.
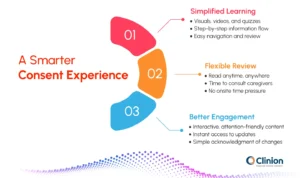
eConsent enhances their experience through:
Simplified Learning
eConsent presents study information in a structured and easy-to-understand format. Visual aids, short explainer videos, and stepwise content help participants clearly understand procedures, expectations, and their role in the study, while allowing them to revisit sections whenever needed.
Flexible Review
Participants can review the consent information at their own pace, outside the clinical setting. This flexibility gives them time to reflect, discuss participation with caregivers or family members, and make informed decisions without the pressure of onsite visits.
Better Engagement
Interactive elements keep participants attentive and involved throughout the consent process. With immediate access to updated versions and clear acknowledgment of changes, participants stay informed without confusion, supporting transparency and trust.
How eConsent Supports Enrollment and Retention
Recruitment teams often face challenges such as participant misunderstandings, complex forms, or limited accessibility, which can slow enrollment and increase dropouts.
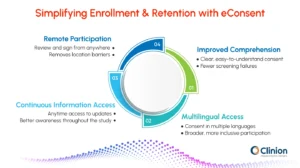
eConsent assists by:
Reducing screen failures through better participant comprehension
When participants clearly grasp what is expected, they are less likely to be disqualified or fail screening.
Allowing multilingual access to materials
eConsent platforms can provide consent forms in multiple languages, enabling diverse patient populations to participate. This expands the reach of clinical trials and ensures inclusivity.
Helping participants stay informed throughout the study
Participants can access updated consent forms and study-related information at any time. Continuous access helps them remain aware of procedures, responsibilities, and any changes in the study.
Providing remote access for patients who live far from sites
Participants can review and sign consent forms from home or other convenient locations. This flexibility removes geographical barriers and encourages participation in decentralized or hybrid trials.
Operational Advantages for Study Teams
Sites and sponsors experience clear operational benefits when using a clinical trial eConsent platform. These advantages streamline workflows, reduce errors, and support regulatory compliance.
Administrative Relief
- Automatic version tracking - eConsent systems automatically record each version of a consent form, ensuring that sites always use the latest approved document.
- Centralized storage - All signed forms are stored in a single digital location, making retrieval simple for staff, monitors, or auditors.
- Faster review of signed forms - Electronic submission allows staff to review and verify consent forms instantly instead of waiting for paper processing.
- Fewer missing signatures - Built-in prompts and required fields reduce the risk of incomplete forms, helping staff stay compliant with study requirements.
Improved Oversight
- Real-time audit trails - Every participant action is recorded, allowing sponsors and monitors to track consent progress and resolve queries quickly.
- Instant access to participant status -Staff can immediately see which participants have completed consent, which forms are pending, and any outstanding issues.
- Clear documentation for inspections and monitoring - Digital records simplify audits by providing a ready-to-review history of consents and acknowledgments.
Fewer Errors
- Reduced risk of outdated consent forms - Platforms ensure participants always sign the most current version, preventing compliance issues.
- Timely acknowledgment of protocol amendments - Updates to the study protocol or consent forms are automatically flagged for participants to review and acknowledge.
- Structured workflows for each site - Consistent digital processes guide staff through each step, minimizing variability across sites and reducing administrative burden.
Paper vs eConsent: A Quick Comparison
Requirement | Paper Consent | eConsent |
Version control | Manual tracking | Automatic updates |
Participant comprehension | Highly variable | Improved through multimedia |
Audit readiness | Time consuming | Real-time trails |
Remote access | Not available | Fully accessible |
Storage | Physical files | Centralized digital repository |
What To Look For in an eConsent Platform
Choosing the right eConsent platform can influence participant engagement, compliance, and overall study efficiency.
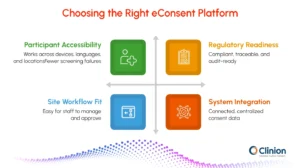
When evaluating options, consider these key areas:
Support Participants Across Environments
- Mobile, tablet, and desktop access - Participants should be able to complete consent forms on any device, allowing flexibility whether they are at home, at a clinic, or on the go.
- Multilingual forms - Providing consent documents in multiple languages ensures inclusivity and supports diverse participant populations.
- Offline availability - Some participants may have limited internet access. Platforms that allow offline review and signing help prevent delays and ensure accessibility.
Align With Your Site Workflows
- Simple staff onboarding - The platform should be intuitive for site staff, requiring minimal training to efficiently manage consents.
- Easy review and countersignature processes - Coordinators, investigators, and other signatories should be able to review, approve, and countersign forms quickly without manual paperwork.
- Role-based permissions - Access controls ensure that staff only see the information relevant to their role, improving security and reducing errors.
Meet Regulatory Standards
- ICH E6(R2) and R3 expectations - eConsent platforms should present study information clearly, maintain traceable documentation of consent, and accurately record participant acknowledgment.
- Digital signature requirements - Platforms must support legally recognized electronic signatures, time-stamped audit records, and identity verification steps to ensure authenticity.
- IRB considerations - The platform should allow submission of interactive or multimedia content, provide clear justification for its use, and ensure participants can ask questions at any time.
Integrate With Your Clinical Systems
- EDC compatibility - Integration with electronic data capture systems ensures consent data flows seamlessly into study databases.
- Single source of truth for consent data - A centralized system reduces duplication and ensures all stakeholders have access to the most up-to-date information.
- Automated sharing of participant status - Real-time updates on who has completed or is pending consent improve study oversight and site efficiency.
Common Misconceptions About eConsent
Many assumptions prevent organizations from adopting eConsent early. Addressing these misconceptions helps teams make informed decisions.
- eConsent is not only for decentralized trials
- eConsent does not reduce opportunities for patient questions
- eConsent does not eliminate the need for human interaction
- eConsent does not complicate regulatory submissions
- eConsent is not just a digital signature
In reality, eConsent strengthens patient communication and provides more clarity for IRBs and regulators.
Conclusion
eConsent in clinical trials has become a foundational component of modern research. It helps participants understand complex information, gives sites reliable tools to manage consent documentation, and improves oversight across studies of all sizes. As more trials adopt digital workflows, an effective eConsent platform will be essential for improving participant experience and operational efficiency.
Clinion eConsent:
Clinion eConsent simplifies and digitizes the informed consent process, enabling participants to review, understand, and sign consent forms anytime, anywhere. With interactive, multilingual, and device-agnostic access, it improves comprehension and engagement while supporting remote participation. Built to align with site workflows and regulatory expectations, Clinion eConsent ensures secure, traceable, and compliant consent management throughout the study lifecycle.

Abriti Rai writes on the intersection of AI, automation, and clinical research. At Clinion, she develops content that simplifies complex innovations and highlights how technology is shaping the next generation of data-driven clinical trials.
FAQS
Frequently Asked Questions
An eConsent platform is a digital system designed to present study information, capture participant consent, and manage documentation electronically. Unlike traditional paper-based eConsent forms, these platforms can include multimedia content, interactive quizzes, and remote signing capabilities, improving comprehension and workflow efficiency.
eConsent in clinical trials provides a fully traceable audit trail, time-stamped signatures, and version control. These features make it easier for sponsors, monitors, and IRBs to review consent data, ensuring adherence to 21 CFR Part 11, HIPAA, and global regulations.
Yes. Electronic informed consent in clinical trials reduces errors associated with manual data entry. Platforms automatically capture participant responses, track acknowledgments, and update consent versions, ensuring all consent forms are accurate and current.
A clinical trial eConsent system allows participants to review and sign forms remotely from any location. This capability expands access for patients who cannot visit a study site, supporting hybrid or fully decentralized trial models while maintaining compliance.
An eConsent solution includes encryption of consent data, role-based permissions, secure logins, and audit trails. These measures protect participant information and maintain privacy, ensuring the platform aligns with regulatory and organizational security standards.
eConsent forms can be presented in multiple languages, allowing global participants to understand study requirements fully. Multilingual capabilities ensure inclusivity and reduce miscommunication, which can improve enrollment and retention rates.
A good eConsent platform provides intuitive navigation, simple onboarding, automated version updates, and easy countersignature workflows. These features reduce administrative burden and allow coordinators to focus on participant engagement rather than paperwork.
By incorporating videos, interactive modules, and stepwise explanations, electronic informed consent clinical trials ensure participants grasp complex study information. This engagement improves comprehension, reduces screening failures, and empowers participants to make informed decisions.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


