Insights / Blog / eSource
eSource in Clinical Trials: Why Electronic Source Data is Now Essential for Modern Research
- Abriti Rai
- January 21, 2026

On this Page
- Summary
- What is eSource in Clinical Trials?
- eSource vs. Traditional Source Data
- Common Forms of eSource in Clinical Trials
- How eSource Works in Clinical Trials
- Benefits of eSource in Clinical Trials
- eSource and Its Growing Influence on Real-World Data (RWD)
- Best Practices for Implementing eSource in Clinical Trials
- Key Challenges in eSource Technology Adoption
- The Future of eSource in Clinical Trials
- References
- Summary
- What is eSource in Clinical Trials?
- eSource vs. Traditional Source Data
- Common Forms of eSource in Clinical Trials
- How eSource Works in Clinical Trials
- Benefits of eSource in Clinical Trials
- eSource and Its Growing Influence on Real-World Data (RWD)
- Best Practices for Implementing eSource in Clinical Trials
- Key Challenges in eSource Technology Adoption
- The Future of eSource in Clinical Trials
- References
Summary
eSource in clinical trials involves capturing data directly in electronic format at its source. This approach reduces errors associated with manual transcription, accelerates data availability for sponsors and monitors, and preserves a clear, auditable record. By supporting secure access and adherence to regulatory standards, eSource enables more transparent, efficient, and well-managed clinical trials.
The adoption of the eSource solution has transformed clinical trials, with 83% of sites reporting improved data quality. Far from a niche innovation, eSource in clinical trials now underpins modern study design, enabling accurate data capture, smoother site workflows, and flexible trial models such as decentralized and hybrid studies.
What is eSource in Clinical Trials?
Electronic Source (eSource) in clinical trials refers to any trial source data that is initially recorded in an electronic format. Unlike traditional handwritten or paper-based documentation, eSource ensures that the electronic version is the primary and original record.
This includes digital capture of clinician observations, patient assessments, lab outputs, device readings, and operational trial records, all created and stored electronically.
eSource vs. Traditional Source Data
For decades, paper has been the default in clinical research. Traditional documentation introduces challenges such as handwritten notes, double data entry, inconsistent formats, and a heavy reliance on on-site monitoring.
The shift from manual to digital source data is more than a technological change; it fundamentally improves data reliability and trial efficiency.
Common Forms of eSource in Clinical Trials
eSource in clinical trials can take many forms depending on the study’s design, endpoints, and patient population. Over time, several formats have become widely adopted across therapeutic areas and study phases. These approaches streamline data capture, reduce errors, and enable the creation of richer datasets for analysis.
eSource Type | Description / Benefit |
Direct Data Capture (DDC) | Digital forms entered directly by clinicians or site staff, removing the need for later transcription into electronic data capture (EDC) systems. |
EHR Integration | Automatic or semi-automatic extraction of clinical data captured during routine care, reducing redundancy and errors. |
eCOA / ePRO | Electronic patient-reported outcomes (ePRO) and assessments that minimize recall bias and support real-time reporting. |
Wearables & Sensors | Devices that continuously track physiological metrics such as heart rate, mobility, or glucose. |
eConsent | Electronic consent solutions with interactive tools, videos, and guided explanations to enhance participant understanding. |
Study Devices / Apps | Custom digital tools designed specifically for trial endpoints or patient monitoring. |
These formats empower sponsors, sites, and participants by creating more accurate, accessible, and comprehensive datasets. They also remove many procedural barriers inherent in traditional source data collection, enabling trials to run more efficiently while maintaining regulatory compliance and high data integrity.
Rare or Emerging Forms of eSource
New technologies are expanding the possibilities of eSource in clinical trials. Although adoption is still limited, these innovations are already shaping advanced clinical data capture:
- Voice-to-text clinician assistants
- IoT-based home monitoring systems
- Ingestible sensors and smart pill bottles
- Passive smartphone sensor data (digital biomarkers)
- Real-time AI-driven diagnostic feeds
These advanced approaches broaden what’s possible in both traditional clinical trials and digital health research.
How eSource Works in Clinical Trials
eSource captures clinical trial data directly at its origin, streamlining workflows and improving data integrity across sites and studies.
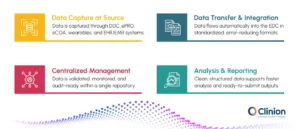
1. Data Capture at Source
Clinicians record information through Direct Data Capture (DDC), ensuring accuracy at the moment of entry. Patients share outcomes using ePRO and eCOA applications that streamline participation. Wearables and sensors continuously track physiological or behavioral metrics for richer datasets. EHR and EMR systems contribute relevant clinical information that enhances completeness and context.
2. Data Transfer & Integration
All captured data moves smoothly into the EDC software without manual handling. Standardized formats reduce transcription errors and maintain consistency across sources. Automated mapping ensures each data point aligns with the study structure and protocol requirements.
3. Centralized Management
The EDC serves as a single, unified repository for all study data. Built-in validation and monitoring tools keep data accurate, compliant, and audit-ready. Remote dashboards provide real-time oversight so teams can track progress across all sites.
4. Analysis & Reporting
Clean, structured data supports faster and more reliable analysis. Queries are resolved quickly due to clear traceability and automated checks. Data is immediately usable for regulatory submissions without additional transcription or reconciliation.
By capturing, integrating, and managing data digitally from the start, eSource accelerates clinical trials while ensuring accuracy, traceability, and regulatory compliance.
Benefits of eSource in Clinical Trials
Implementing eSource delivers measurable benefits across data capture, site workflows, and trial monitoring:
Improved Data Quality and Accuracy
Capturing data directly at the source ensures structured, validated, and time-stamped entries. Automated logic and built-in edit checks reduce errors and create a more complete and reliable dataset from the start.
Enhanced Efficiency Across Sites and Studies
Digital workflows streamline documentation and remove repetitive tasks, enabling sites to manage multiple studies effectively while maintaining productivity.
Reduced Protocol Deviations and Monitoring Workload
Guided digital processes minimize missed assessments and incorrect entries. Remote monitoring allows clinical research associates to review source data efficiently without frequent site visits.
Real-Time Oversight
Continuous access to trial data supports early detection of issues, proactive risk-based monitoring, and faster resolution of queries.
Stronger Compliance and Data Integrity
Automated audit trails and controlled access enforce regulatory standards, including ALCOA+ principles, while ensuring the integrity of collected data.
Lower Operational Costs
Eliminating manual transcription, printing, and archiving reduces overhead and contributes to long-term savings for trial operations.
Better Patient Experience and Engagement
Digital diaries, wearables, and remote assessments reduce patient burden and help sustain engagement throughout the study.
eSource and Its Growing Influence on Real-World Data (RWD)
eSource in clinical trials captures data electronically at the point of origin, from electronic health records, wearables, and patient apps, rather than through manual transcription. This direct data capture reduces errors and improves data quality, creating a more reliable foundation for Real-World Data (RWD) databases.
eSource also bridges the gap between controlled trials and real-world practice. By pulling data directly from patients' existing health records and digital tools, trial data becomes more representative of routine care. This makes it easier to integrate trial findings with post-market RWD for safety monitoring and effectiveness studies, while enabling seamless longitudinal tracking beyond trial completion.
Additionally, eSource promotes standardization through formats like FHIR, allowing researchers to combine clinical trial data with broader RWD ecosystems more effectively. This creates a data continuum from trials to post-market surveillance, supporting better regulatory decisions and a more comprehensive understanding of treatment outcomes across the entire lifecycle of medical interventions.
Best Practices for Implementing eSource in Clinical Trials
A well-planned approach helps eSource deliver value without creating additional pressure on study teams.
Start with clear objectives for data quality and efficiency
Define what success means, such as fewer deviations, faster entry, reduced queries, or improved monitoring readiness.
Align stakeholders early
Sites, monitors, and sponsors should agree on new workflows and responsibilities before implementation begins.
Ensure strong data security and privacy
Use encryption, controlled access, and compliant hosting as foundational elements of the system.
Design workflows that reduce site burden
Keep interfaces simple and create role-specific pathways to encourage smooth adoption.
Plan for scalable integration with EDC and EHR systems
Use standardized exchange frameworks such as HL7 and FHIR to support long-term interoperability.
Provide thorough training and continuous support
Hands-on sessions and ongoing guidance help teams stay confident and consistent.
Run a pilot before full rollout
Start with a controlled pilot to refine processes and address challenges before broader deployment.
Key Challenges in eSource Technology Adoption
Even with its advantages, eSource adoption still faces barriers across sites and sponsors.
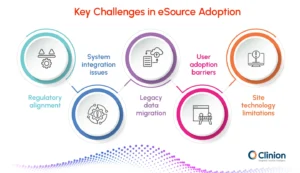
Regulatory alignment
Ensuring compliance with ALCOA+, 21 CFR Part 11, and regional data laws remains complex.
Validation, audit trails, identity controls, and documentation must all meet regulatory expectations. Any gaps can slow approvals or create rework for sponsors and sites.
System integration issues
eSource must connect smoothly with EDC, EHR systems, and existing site workflows.
Fragmented technology and inconsistent data standards often disrupt this alignment. Poor integration leads to duplicate entries, delays, and inconsistent datasets.
Legacy data migration
Paper records and older digital systems create challenges during eSource transition. Digitizing, validating, and standardizing legacy data requires time and careful oversight. Without proper migration, historical data may lose accuracy or traceability.
User adoption barriers
Site staff may be reluctant to shift from familiar paper or EDC-based workflows. Training and clear guidance are essential to build comfort and confidence. Low adoption can limit the value of eSource, even if the technology is strong.
Site technology limitations
Sites differ widely in hardware quality, connectivity, and IT support. Limited infrastructure can affect real-time data capture and system reliability. Ensuring consistent performance across diverse environments is a key challenge.
The Future of eSource in Clinical Trials
- AI will shift from detection to intelligent decision-making eSource systems will evolve from simply flagging anomalies to interpreting patterns, prioritizing risks, and suggesting corrective actions. This will help monitors and data managers resolve issues faster and reduce the need for manual oversight in low-complexity cases.
- Unified digital ecosystems will support fully hybrid and adaptive trials Instead of fragmented tools, eSource will integrate wearables, telehealth, home diagnostics, ePRO, and site workflows into a single connected environment. This will enable fluid transitions between on-site and remote participation while maintaining consistent data quality.
- Interoperability will become plug-and-play across healthcare and research systems With advancing FHIR standards, eSource will communicate seamlessly with EHRs, national health databases, and claims systems. This will reduce setup time for multi-site studies and improve the depth and continuity of patient data used in trials.
- Regulatory frameworks will increasingly expect digital-first source data Agencies are moving toward clearer guidance that positions eSource as the preferred method for capturing clinical evidence. As adoption becomes standard practice, paper workflows will be considered exceptions rather than the norm.
- Continuous real-world data (RWD) will become part of routine evidence generation eSource will increasingly draw from longitudinal patient data, device streams, routine care, pharmacy data, and patient apps. This will strengthen post-marketing surveillance and support ongoing safety, effectiveness, and outcomes research.
eSource has become a practical standard in modern clinical trials, improving data quality, reducing site burden, and enabling more flexible study designs. As regulatory agencies increasingly recognize and support digital source data, its role in clinical research will only continue to expand.
Clinion eSource: The Smarter Way to Capture Source Data
Clinion eSource removes the limitations of traditional workflows by allowing site users to capture source data directly through a secure mobile app at the point of care. Direct Data Capture eliminates duplicate entry and reduces data-cleaning cycles, delivering faster study conduct and lower monitoring costs. With real-time quality checks, continuous oversight, and full compliance with FDA 21 CFR Part 11, GDPR, and ICH-GCP, Clinion eSource ensures a reliable, inspection-ready source of truth for every trial.
References
FDA Guidance for Industry Electronic Source Data in Clinical Investigations
Esource Clinical Trials Assets
Accelerating the Adoption of eSource in Clinical Research: A Transcelerate Point of View - PMC
Defining methods to improve eSource site start-up practices - ScienceDirect
eSource for clinical trials: Implementation and evaluation of a standards-based approach in a real world trial - ScienceDirect

Abriti Rai writes on the intersection of AI, automation, and clinical research. At Clinion, she develops content that simplifies complex innovations and highlights how technology is shaping the next generation of data-driven clinical trials.
FAQS
Frequently Asked Questions
An electronic source includes any original trial data that is captured or stored in a digital format. This includes eCRFs, ePRO, eConsent, connected devices, wearables, and direct data capture tools.
Paper creates delays, errors, and extra monitoring work. eSource gives cleaner data, real-time visibility, and faster decision-making. It removes transcription and simplifies site operations.
It reduces paperwork and duplicate entry. Sites spend less time recording and correcting data and more time focusing on patients. Structured digital workflows guide sites and prevent common mistakes.
Not at all. On-site trials also benefit. eSource improves accuracy, reduces data queries, and shortens review cycles in every trial model.
Sponsors should look for strong EDC integration, flexibility for different study designs, device and form compatibility, audit-ready traceability, and automation that reduces manual effort.
Digital source data produces a clear, traceable record that aligns with GCP, ALCOA+, and FDA and EMA expectations for reliability and data integrity.
Monitors can review source data in real time and focus on targeted checks. This reduces travel, effort, and cost while strengthening oversight.
Data is cleaner from the start. Automatic validations and fewer discrepancies shorten query cycles and allow teams to lock databases faster.
Clinion provides eSource as part of a unified platform. Data flows directly into EDC without middleware, manual syncing, or multiple systems. Sites work in one connected environment, which reduces errors and setup complexity.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


