Insights / Blog / RTSM
RTSM in Clinical Trials: A Guide to Randomization and Trial Supply Management
- Abriti Rai
- January 8, 2026

On this Page
- Summary
- What is RTSM in Clinical Trials?
- Why Do Trials Need Randomization and Trial Supply Management (RTSM)?
- How Randomization Works in an RTSM
- Clinical Trial Supply Management in RTSM
- What an RTSM System Should Support
- Benefits of Using RTSM in Clinical Trials
- How to Choose the Right RTSM for Your Clinical Trial
- RTSM Compliance Best Practices
- The Future of RTSM
- Summary
- What is RTSM in Clinical Trials?
- Why Do Trials Need Randomization and Trial Supply Management (RTSM)?
- How Randomization Works in an RTSM
- Clinical Trial Supply Management in RTSM
- What an RTSM System Should Support
- Benefits of Using RTSM in Clinical Trials
- How to Choose the Right RTSM for Your Clinical Trial
- RTSM Compliance Best Practices
- The Future of RTSM
Summary
Randomization and Trial Supply Management (RTSM) is closely tied to how clinical trials enroll participants and manage study medication. These activities shape the flow of a trial and ensure that each step follows the approved protocol. Understanding these parts of a study helps readers appreciate why RTSM is widely used across research today.
What is RTSM in Clinical Trials?
RTSM, or Randomization and Trial Supply Management, is a system used to coordinate how participants are assigned to treatment groups and how study medication is distributed throughout a clinical trial. It brings together two important functions:
Randomization, which ensures every participant is allocated to a treatment arm based on predefined rules
Clinical trial supply management, which controls how the study drug is packaged, shipped, stored, and dispensed at each site
In most trials, these processes need careful oversight because they influence study integrity, patient safety, and protocol adherence. RTSM helps automate these activities so that the trial remains consistent and compliant as it progresses.
RTSM is also used to enforce rules defined in the protocol, monitor supply levels at each site, and maintain the blind when required. By managing these elements in a central system, studies are able to reduce manual work and avoid errors that could affect patient allocation or medication handling.
Clinical Trial Response Systems: Comparing RTSM, IRT, IWRS, and IxRS
In clinical trials, multiple systems support patient randomization, treatment assignment, and trial logistics. Terms like IRT, IWRS, IxRS, and RTSM are often used together, and sometimes interchangeably; however, they differ in their operational scope and functional depth. The table below summarizes how these commonly used systems vary in purpose and coverage:
Term | Primary Role in Clinical Trials | Scope of Randomization | Scope of Supply Management | Typical Use Case |
IRT (Interactive Response Technology) | Broad term for systems that support treatment assignment workflows | Patient randomization and treatment allocation | Not designed for end-to-end supply control | Used when the primary need is controlled treatment assignment |
IWRS (Interactive Web Response System) | Web-based interface for executing randomization and basic drug dispensing | Centralized, protocol-driven randomization | Limited site-level dispensing support | Common in studies with simpler supply models |
IxRS (Interactive Response System) | Combines web and voice technologies for trial logistics | Centralized randomization via web or phone | Limited, typically reactive supply handling | Used when sites require phone-based access in addition to web |
RTSM (Randomization and Trial Supply Management) | Integrated management of randomization and full trial supply operations | Protocol-driven randomization across all sites | End-to-end planning, forecasting, inventory, resupply, and reconciliation | Preferred for complex, multi-site and global trials with active supply chains |
Why Do Trials Need Randomization and Trial Supply Management (RTSM)?
Randomization plays a key role in clinical trials by helping ensure that treatment groups are comparable, which strengthens the scientific integrity of the study. Similarly, careful management of study supplies helps make sure that each participant receives the right medication when needed, supporting both patient safety and smooth trial operations.
RTSM supports clinical trials by:
- Maintaining consistent treatment allocation across participants and sites
- Preventing accidental unblinding, protecting study integrity
- Monitoring inventory levels at each trial site in real time
- Avoiding drug shortages or wastage through efficient supply management
- Enforcing protocol rules for dosing, scheduling, and visit requirements
- Creating visibility for sponsors, CROs, and supply teams, enabling proactive decision-making
Randomization and trial supply management (RTSM) work together to ensure that trials run reliably. These processes give sponsors, CROs, and study teams the oversight they need to keep trials on track and compliant with the protocol.
How Randomization Works in an RTSM
Randomization in RTSM is guided by the study protocol, ensuring each participant is assigned to a treatment group according to pre-established rules. The process involves multiple methodologies, defined roles, and a structured workflow to maintain accuracy and compliance.
Note: In clinical trials, each treatment arm represents a group of participants receiving the same therapy, dose, or placebo. Randomizing participants into these arms helps maintain balanced comparisons and reduces bias.
Types of Randomization in RTSM Systems
Different trials may use different randomization approaches depending on the study design and objectives.
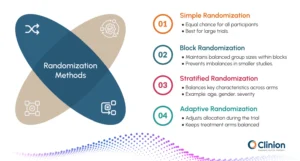
Common methodologies include:
Simple Randomization
Each participant has an equal chance of being assigned to any treatment arm. This method is straightforward and works well for larger trials, but may occasionally lead to imbalances in smaller studies.
Block Randomization
Participants are assigned to predefined blocks to ensure balanced group sizes throughout the trial. This approach prevents large imbalances between treatment arms, which can be particularly useful in smaller or multicenter studies.
Stratified Randomization
Participants are grouped based on key characteristics such as age, gender, or disease severity before being randomized. This ensures that important factors are evenly distributed across treatment arms, improving the reliability of results.
Adaptive Randomization
Allocation probabilities can change as the trial progresses, based on accumulating data. For example, the system might assign more participants to a treatment arm showing better responses, while maintaining overall balance and statistical validity. This method is often used in complex or exploratory trials to optimize outcomes and resource use.
Who Creates the Randomization List?
The randomization list is a critical part of ensuring a trial is unbiased and follows the study protocol. It is prepared by trained professionals with expertise in study design, statistics, and RTSM systems, including:
Biostatisticians
Experts in clinical trial statistics design randomization schemes that maintain balance and account for patient characteristics.
Randomization Specialists
Professionals focused specifically on randomization processes ensure the methods are implemented correctly in RTSM.
Validated Statistical Software Teams
Technical teams use certified software tools to generate and validate the randomization list, ensuring accuracy and compliance.
Before the list is used in the RTSM, the sponsor reviews and formally approves it. This review confirms that the list aligns with the study protocol and trial design, ensuring both regulatory compliance and scientific integrity.
Who Imports the Randomization List into the RTSM System?
Once the randomization list is finalized and approved, it must be integrated into the RTSM system. This step ensures that the automated randomization workflow operates correctly and aligns with the trial protocol. Typically, the task is handled by:
- RTSM Provider’s Technical Configuration Team
Experts who understand the system’s architecture and randomization functionality carefully upload the list and configure any system settings needed to implement the correct randomization rules. - Study Data Management Personnel
Team members are responsible for ensuring data integrity and compatibility. They verify that the imported list matches the approved protocol specifications and that the system can track all assignments accurately. - Study-Specific RTSM Administrator
A designated individual oversees the process from the sponsor or CRO side, coordinating between technical teams and investigators to ensure the list is correctly implemented.
Before the trial goes live, the team performs validation checks to confirm:
- All participants will be allocated according to the protocol.
- The blind is maintained for those roles that should not see treatment assignments.
- System-generated assignments match the approved randomization plan.
This structured process ensures accuracy, reduces human error, and allows the trial to begin with confidence in both patient safety and data integrity.
How RTSM Implements Subject Randomization
Once a clinical trial is active, RTSM automates patient allocation in a stepwise and controlled manner. This ensures that every participant is assigned correctly, the study blind is maintained, and the trial protocol is followed precisely.
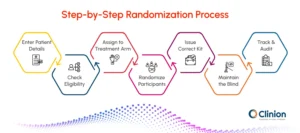
Here’s a detailed overview of the process:
Enter Subject Details
Study site staff input participant information such as demographics, inclusion/exclusion criteria, and any stratification factors required by the trial design. RTSM captures these details in real time and prepares the system for the correct treatment assignment.
Check Eligibility
The system automatically verifies that the participant meets all protocol criteria before allocation. Any discrepancies or ineligible entries trigger alerts, preventing incorrect assignments and ensuring patient safety.
Assign to Treatment Arm
Using the pre-approved randomization list or algorithm, RTSM assigns each participant to the appropriate treatment arm. Each arm represents a group of participants receiving the same therapy, dose, or placebo, allowing fair comparison between groups.
Issue Correct Kit
RTSM selects and labels the investigational product (IP) kit or dose according to the assigned treatment arm. This ensures participants receive the right medication at the right time and reduces human errors in supply allocation.
Maintain the Blind
Access to treatment details is restricted based on user roles. Investigators and designated personnel see only the information necessary to conduct visits and dispense medication. Emergency unblinding procedures are built in for patient safety and controlled access.
Track & Audit
Every action is recorded in RTSM, creating a detailed audit trail for regulatory compliance and quality assurance. Real-time dashboards allow sponsors, CROs, and site teams to monitor enrollment, treatment distribution, and protocol adherence efficiently.
Blinding and Patient Safety in RTSM
Blinding is a method that is used in clinical trials to prevent bias. It ensures that certain individuals, such as participants, investigators, or analysts, do not know which treatment a participant is receiving.
By limiting who can see this information, the study remains objective, and outcomes are evaluated fairly. RTSM plays an important role in maintaining this protection by controlling access to treatment details, managing kit visibility, and enforcing protocol-specific blinding rules.
Types of Blinding Managed Through RTSM
Single-Blind
Participants are unaware of which treatment they receive. Depending on the study design, some site staff may know the assignments.
Double-Blind
Neither participants nor investigators know the treatment allocation. RTSM restricts treatment details and manages kit labels so that the blind is preserved throughout the trial.
Triple-Blind
Participants, investigators, and data analysts do not have access to treatment information. RTSM masks all treatment-related data until the database is locked.
Emergency Unblinding
If a medical emergency requires knowing a participant’s treatment, RTSM enables secure, role-restricted unblinding. Only authorized personnel are permitted to perform this task, and the system records every action to ensure full accountability.
Clinical Trial Supply Management in RTSM
Once participants are randomized, clinical trials depend on the precise coordination of study medication across sites. Trial supply management focuses on ensuring that the right investigational product (IP) is available, in the correct quantity, and at the right time throughout the study lifecycle.
In clinical trials, supply chains are rarely static. Enrollment rates can change, sites may activate at different times, and dosing schedules often vary by participant. RTSM helps manage this complexity by centralizing supply planning, tracking, and distribution within a single system.
Within RTSM, trial supply management supports multiple stages of the supply lifecycle, including:
- Packaging and labeling of study medication
- Shipment of supplies to depots and clinical sites
- Tracking inventory levels at each location
- Managing expiries, returns, and destruction
- Allocating medication based on visit schedules and dosing rules
By coordinating these activities centrally, RTSM reduces reliance on manual tracking and minimizes the risk of supply disruptions.
Key Steps Involved in Trial Supply Management
Clinical trial supply management follows a structured sequence to ensure medication is available throughout the study. While details vary by protocol, most trials follow these core steps, supported centrally through RTSM.

Supply Planning
Before the trial begins, supply teams estimate medication needs based on enrollment targets, dosing schedules, site locations, and treatment arms. RTSM supports this planning by aligning supply assumptions with protocol rules.
Packaging and Labeling
Investigational products are packaged and labeled according to regulatory and protocol requirements. RTSM maintains traceability by linking each kit or batch to the study without revealing treatment details in blinded trials.
Distribution to Depots and Sites
Study medication is shipped to central depots and clinical sites based on activation timelines. RTSM tracks shipment status and receipt to maintain visibility across locations.
Site Inventory Management
Once supplies reach a site, RTSM monitors inventory levels in real time. The system tracks quantities, expiry dates, and storage status to prevent shortages or unusable stock.
Dispensing at Study Visits
At each visit, RTSM assigns the correct kit or dose based on the participant’s randomization and visit schedule. This ensures protocol-compliant dispensing while maintaining the blind.
Resupply and Reconciliation
RTSM automatically triggers resupply when inventory drops below predefined thresholds. It also supports returns, reconciliation, and destruction of unused or expired medication.
Ongoing Monitoring and Adjustments
As enrollment and trial activity change, RTSM helps teams adjust supply strategies. This includes responding to protocol amendments, enrollment shifts, or site-level variations.
How Trial Supply Controls Support Patient Safety
Clinical trial supply management plays a direct role in protecting patient safety. Errors in medication handling, dosing, or availability can affect both participant well-being and protocol compliance. RTSM helps reduce these risks by applying automated checks at key points in the supply workflow.
Within an RTSM system, safety-focused controls are built into how study medication is tracked and dispensed.
Trial Supply Management helps protect patient safety by:
- Preventing the use of expired or temperature-compromised medication
- Ensuring only protocol-compliant doses can be assigned and dispensed
- Restricting access to treatment arms based on user role and trial design
- Monitoring site inventory to prevent stockouts that could interrupt treatment
By enforcing these rules centrally, RTSM reduces reliance on manual checks and helps ensure that participants receive the correct medication at every visit.
What an RTSM System Should Support
An RTSM system acts as the operational backbone of a clinical trial, coordinating participant flow and study medication handling according to the protocol. To do this effectively, it must support the following core activities.
Screening and Enrolment
RTSM supports participant screening by capturing eligibility criteria and confirming whether a subject can be enrolled. Once eligibility is verified, the system controls enrolment to prevent over-enrolment and ensures subjects are only randomized when protocol conditions are met.
Visit Scheduling
RTSM aligns participant visits with the study schedule defined in the protocol. It tracks visit windows, enforces visit order, and links each visit to the correct dosing or supply actions, reducing the risk of missed or out-of-window visits.
Drug Assignment
Based on the approved randomization logic, RTSM assigns participants to the correct treatment arm and links them to the appropriate study medication. The system ensures accurate dispensing while maintaining blinding, even in complex multi-arm or adaptive trials.
Re-supply Management
RTSM continuously monitors inventory levels at each site and triggers resupply based on predefined thresholds. This helps prevent drug shortages, avoids over-stocking, and ensures uninterrupted treatment for enrolled participants.
Reporting and Analytics
RTSM provides real-time visibility into enrolment status, treatment allocation, and site inventory. Standard and custom reports help sponsors and CROs track trial progress, identify risks early, and make informed operational decisions.
Benefits of Using RTSM in Clinical Trials
By centralizing randomization and trial supply activities, RTSM helps studies run with greater control, consistency, and confidence. The benefits extend across study teams, sites, and participants.
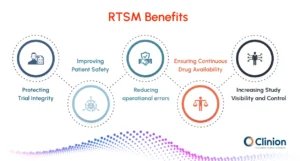
Protecting Trial Integrity
RTSM enforces protocol-defined randomization rules and maintains blinding throughout the study. By reducing manual intervention, it minimizes the risk of allocation bias, unblinding, and protocol deviations that could compromise study results.
Improving Patient Safety
Automated checks ensure that participants receive the correct treatment at the right visit. RTSM helps prevent dosing errors, blocks expired or unsuitable medication, and supports controlled emergency unblinding when safety concerns arise.
Reducing Operational Errors
By automating enrolment, treatment assignment, and supply handling, RTSM reduces reliance on manual processes. This lowers the risk of data entry mistakes, incorrect dispensing, and miscommunication between study teams.
Ensuring Continuous Drug Availability
Real-time inventory tracking and automated resupply help prevent stock-outs and over-supply at sites. This supports uninterrupted treatment schedules and avoids delays that could impact participant retention.
Increasing Study Visibility and Control
RTSM provides centralized dashboards and reports that give sponsors and CROs real-time insight into enrolment progress, treatment distribution, and site performance. This visibility enables proactive decision-making and faster issue resolution.
How to Choose the Right RTSM for Your Clinical Trial
Selecting an RTSM system is not just a technical decision. It also involves evaluating the vendor behind the platform and their ability to support your study from setup through closeout.
Alignment With Study Design
Every clinical trial has unique requirements based on phase, therapeutic area, and geography. An RTSM should support different randomization strategies, dosing rules, and supply models without forcing protocol compromises. A strong system adapts to the study design rather than requiring the study to adapt to the system.
Coverage Across Randomization and Trial Supply Management
Because RTSM connects patient allocation with medication distribution, the platform should manage both in a single, coordinated workflow. Randomization decisions should automatically drive drug assignment, inventory tracking, and re-supply planning. This reduces operational gaps and helps ensure medication availability keeps pace with enrollment.
Blinding and Patient Safety Capabilities
Blinding requirements can range from simple to highly controlled. An RTSM should support role-based access, controlled visibility of treatment details, and secure emergency unblinding processes. Equally important are safety safeguards such as blocking expired or temperature-compromised kits and preventing incorrect dosing or treatment assignment.
Vendor Experience and Therapeutic Expertise
The RTSM vendor plays a key role in successful implementation and ongoing study operations. Experience with different trial phases, therapeutic areas, and global studies can make a significant difference. Vendors with proven RTSM expertise are better equipped to anticipate protocol complexities, manage mid-study changes, and support compliance expectations.
Integration With Other eClinical Systems
RTSM often works alongside EDC, eCOA, CTMS, and other clinical systems. The vendor should support reliable integrations that minimize manual data handling and reconciliation. Well-integrated systems help study teams maintain consistency across platforms and reduce operational friction.
Scalability and Global Support
As trials expand across regions or increase in enrollment, the RTSM and its vendor must scale accordingly. This includes support for multiple depots, regional regulations, time zones, and language requirements. A vendor with global operational support helps maintain continuity as trial complexity grows.
Implementation, Validation, and Ongoing Support
Beyond functionality, consider how the vendor manages system setup, validation, training, and ongoing support. Clear implementation processes and responsive support teams can directly impact trial timelines. Reliable vendor support ensures that protocol amendments, supply adjustments, and enrollment changes are handled smoothly throughout the study lifecycle.
RTSM Compliance Best Practices
Ensuring compliance is critical in clinical trials, and RTSM systems play a central role in maintaining adherence to regulatory standards throughout the study.
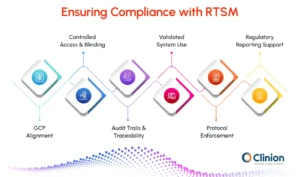
Good Clinical Practice (GCP) Alignment
RTSM enforces protocol-defined rules consistently across sites and participants, helping sponsors meet GCP requirements and maintain study integrity.
Controlled Access and Blinding
Role-based permissions limit access to sensitive treatment information. Investigators, site staff, and analysts only see what they are authorized to, preserving the study's blind and reducing bias.
Audit Trails and Traceability
Every action in the system, from randomization and drug assignment to emergency unblinding, is logged. These detailed records support regulatory inspections and ensure accountability.
Validated System Use
RTSM platforms are validated before study initiation and are managed under formal change control procedures, ensuring consistent performance and compliance throughout the trial.
Protocol Enforcement
Built-in checks prevent errors such as non-compliant dosing, incorrect treatment allocation, and use of expired or temperature-compromised medication. This minimizes risks to patient safety and maintains adherence to study protocols.
Regulatory Reporting Support
RTSM generates structured reports and dashboards that allow sponsors and CROs to monitor compliance in real time, facilitating rapid corrective action if issues arise.
The Future of RTSM
RTSM systems are evolving rapidly to meet the demands of more complex, global, and adaptive clinical trials. The next generation of RTSM emphasizes predictive intelligence, deeper integration, and advanced automation to streamline trial operations while safeguarding patient safety and data integrity.
AI-Driven Forecasting
Artificial intelligence and machine learning are enabling predictive modeling for patient enrollment, treatment allocation, and study drug consumption. Sponsors can anticipate bottlenecks, optimize timelines, and make proactive decisions rather than reacting to issues after they arise.
Predictive Supply Optimization
By analyzing historical and real-time trial data, future RTSM platforms can forecast site-level drug requirements with high precision. This reduces the risk of stockouts or overstock, ensures timely resupply, and decreases wastage, ultimately saving costs and improving trial efficiency.
Unified Clinical Platforms
RTSM is increasingly being integrated with other digital trial systems such as EDC, ePRO, eConsent, and eTMF. This creates a single ecosystem for trial management, offering real-time data visibility, reducing manual handoffs, and simplifying reporting for sponsors, CROs, and site teams.
Enhanced Compliance and Safety
Advanced RTSM platforms use automated checks to enforce protocol rules, maintain blinding, and monitor site-level dosing in real time. This reduces human errors, strengthens regulatory compliance, and enhances overall patient safety.
Scalability for Global and Adaptive Trials
Modern RTSM solutions can manage complex multi-arm, multi-region trials, including adaptive designs, without adding operational complexity. They allow flexible configuration for varying protocols, dosing schedules, and supply chains, making large-scale studies more manageable.
Future-Ready Analytics
The next wave of RTSM emphasizes actionable insights through dashboards, predictive alerts, and AI-driven reporting. Sponsors can identify trends, detect risks early, and make data-driven decisions throughout the trial lifecycle.
RTSM redefines how clinical trials are conducted, turning intricate processes into coordinated, manageable workflows. By centralizing randomization, supply management, and compliance safeguards, it not only streamlines operations but also reinforces the reliability of trial outcomes. Research teams gain confidence knowing that patient safety is preserved, protocol adherence is maintained, and data integrity is protected.
In essence, RTSM enables trials to move forward efficiently, fostering innovation while ensuring that each study contributes meaningful, credible evidence to the field of medicine.
Clinion RTSM
Simplify how randomization and trial supply management are executed across a study. Built with deep integration into Clinion EDC, it enables patient randomization, kit assignment, reassignments, and returns to be managed within a single system. This unified approach reduces the need for multiple logins, minimizes operational handoffs, and helps teams maintain accuracy and compliance throughout the trial. With rapid deployment and flexible configuration, Clinion RTSM supports studies of varying complexity, from simple designs to large, multi-arm global programs.

Abriti Rai writes on the intersection of AI, automation, and clinical research. At Clinion, she develops content that simplifies complex innovations and highlights how technology is shaping the next generation of data-driven clinical trials.
FAQS
Frequently Asked Questions
An RTSM system replaces spreadsheets, emails, and site-level logs with a centralized, validated platform. Unlike manual methods, RTSM automates randomization rules, controls drug assignment, tracks inventory in real time, and enforces protocol compliance consistently across all sites. This significantly reduces human error, improves audit readiness, and ensures uninterrupted patient treatment throughout the trial.
Yes. Modern RTSM solutions are designed to support both simple and complex studies. For early-phase or small trials, RTSM can be configured with straightforward randomization logic and limited supply workflows, while still maintaining blinding, traceability, and compliance. This allows sponsors to scale RTSM usage without adding unnecessary operational burden.
RTSM systems are built to accommodate protocol changes such as updated dosing schedules, new sites, or modified randomization ratios. Changes are implemented under controlled change management and validation processes, ensuring compliance while minimizing disruption to ongoing enrollment and supply distribution.
RTSM provides inspectors with clear, traceable records of randomization, drug assignment, inventory movement, and unblinding events. Comprehensive audit trails, time-stamped actions, and role-based access controls help demonstrate GCP compliance and protocol adherence during audits and inspections.
RTSM systems are well-suited for global studies involving multiple regions, depots, and regulatory requirements. They support regional supply strategies, time-zone differences, language needs, and varying shipment workflows while maintaining centralized oversight of enrollment, inventory, and compliance.
Yes. RTSM is commonly integrated with EDC, ePRO, CTMS, and other clinical systems. These integrations reduce duplicate data entry, ensure consistency across platforms, and allow randomization and supply decisions to align directly with subject data captured in EDC.
For adaptive trials, RTSM can dynamically adjust randomization probabilities, dosing rules, or supply strategies based on predefined protocol logic. This enables sponsors to manage complex study designs while maintaining statistical integrity, blinding, and operational control.
Without RTSM, trials face higher risks of allocation errors, drug shortages, unblinding, protocol deviations, and incomplete documentation. These issues can compromise patient safety, delay timelines, and negatively impact data quality and regulatory outcomes.
RTSM is used by multiple stakeholders, including site staff, supply managers, data management teams, sponsors, and CROs. Each role has controlled access to ensure users see only the information necessary to perform their responsibilities, helping preserve blinding and data integrity.
RTSM is typically implemented during study setup, before site activation and first patient enrollment. Early implementation allows proper validation, testing, and training, ensuring randomization and supply workflows function correctly from day one of the trial.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


