RTSM
The Power of RTSM, Inside Your EDC
The fast, easy, and cost-effective way to manage randomization and trial supply for your studies.
Why RTSM ?
Join 100+ Customers Accelerating Trials Across Phase I to IV
Countries
Subjects
Customers
Clinical Trials
Biotech Studies
FDA Studies
Deep EDC Integration
Eliminate system switching and simplify site workflows by managing randomization and trial supply directly within your EDC system.
Easy to Use
Reduce training time and simplify site workflows with our intuitive, deeply integrated RTSM software design.
Flexible & Scalable
Choose a standalone or fully unified deployment with our flexible and cost-effective RTSM system, built for trials of all sizes.
Features
Key Features of our RTSM Software
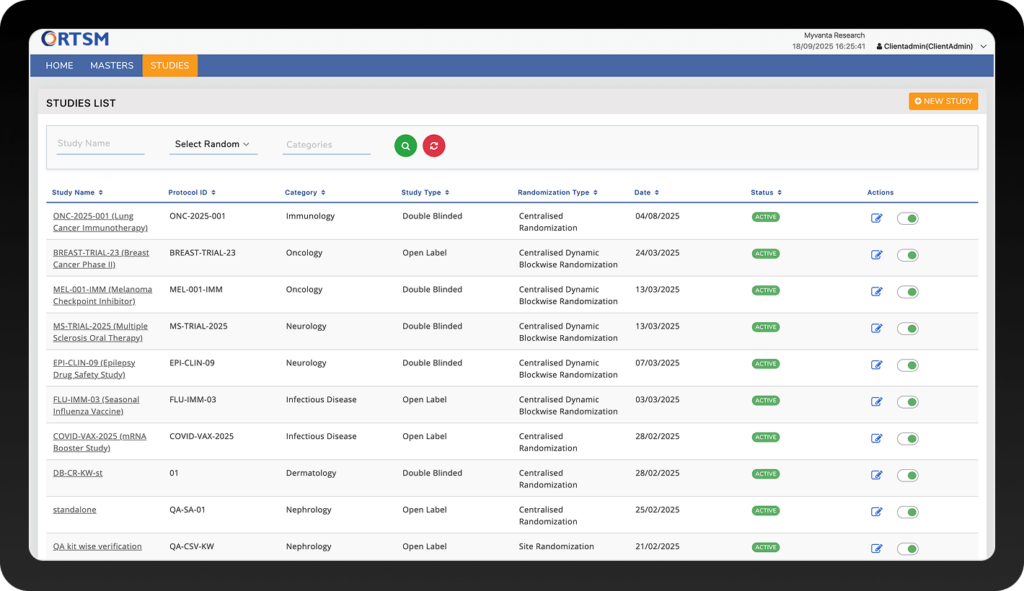
RANDOMIZATION & ENROLLMENT
Ensure Compliant, Error-Free Randomization for Every Single Subject
By automating complex randomization workflows directly within the EDC system, you eliminate the potential for human error, which in turn simplifies site operations and accelerates your trial timelines. Below are the core features that make this possible.
Supports standard designs like simple, dynamic block, cohort, and stratified randomization through the configuration and upload of a statistician-provided Master Randomization List (MRL). Our randomisation and trial supply management architecture is also capable of supporting more complex dynamic allocation methods as required by the protocol.
Provides a validated User Acceptance Testing (UAT) environment that is a direct replica of the production environment. In this UAT space, the study team can conduct comprehensive simulations of all randomization scenarios, enroll test subjects, and verify the randomization logic against the protocol to ensure all aspects of the system behave exactly as expected before go-live.
This is one of the core strengths of our integrated RTSM-EDC platform. Randomization is triggered only when the subject has met all eligibility criteria, and the required data is entered and validated within the EDC. This ensures protocol adherence and eliminates the risk of premature randomization.
Supports re-randomization in complex study designs, such as crossover and re-treatment scenarios. The system allows for multiple randomization events per subject when configured, with eligibility criteria and treatment arms managed according to protocol rules.
Provides a highly flexible unblinding process, configurable for either direct investigator-initiated action or a multi-step Project Manager approval workflow. Every unblinding event is secured by an electronic signature and fully documented in the audit trail to ensure complete transparency and regulatory compliance.
Supports modification of treatment allocation ratios mid-study in alignment with protocol amendments. These changes can be implemented through controlled configuration updates, ensuring continuity and compliance across ongoing studies.
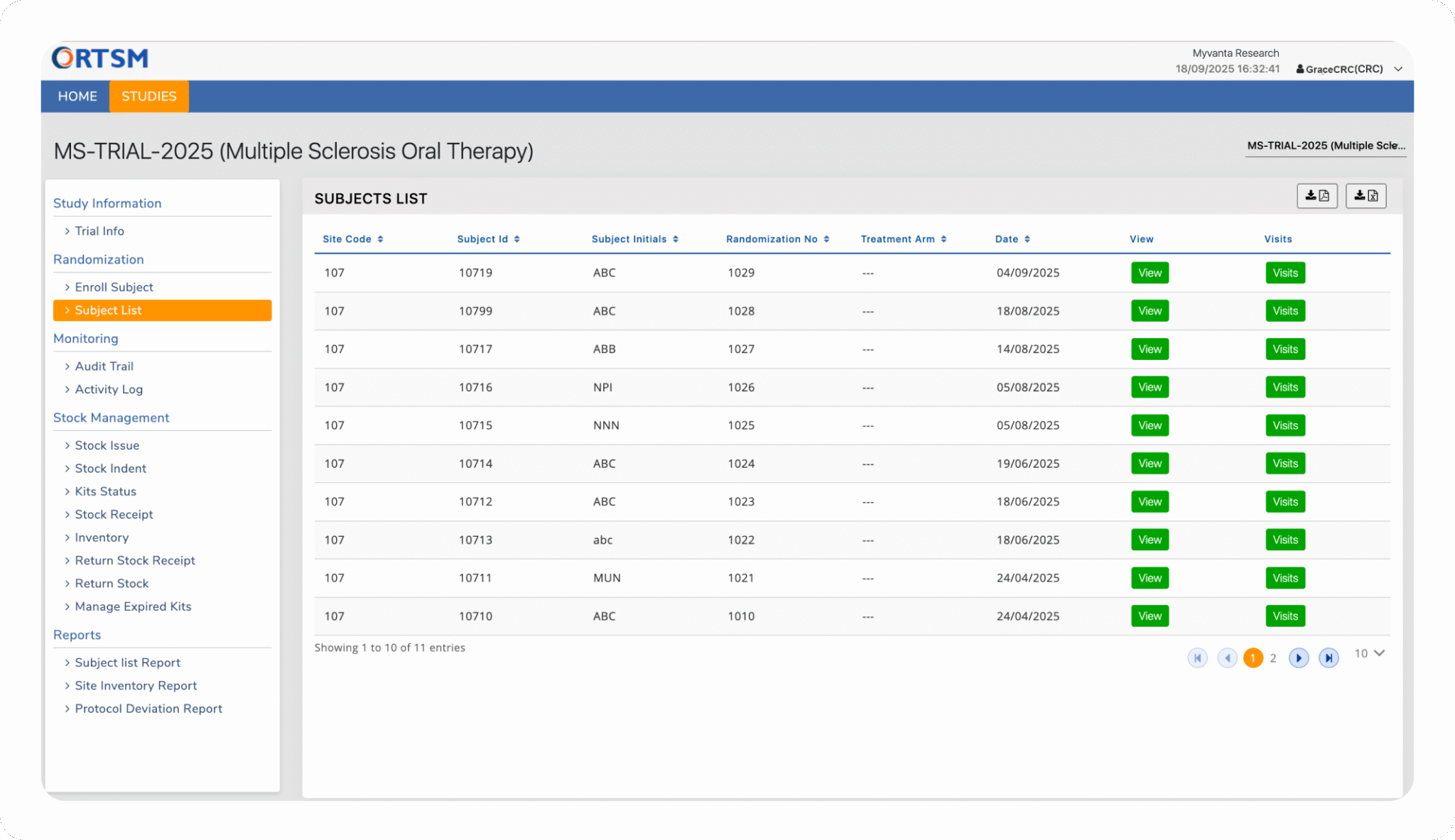
SUPPLY CHAIN MANAGEMENT
Maintain Complete Control Over Your Global Clinical Supply Chain
Our RTSM Software is a centralized platform that gives you end-to-end visibility and control over your entire supply chain, from every depot to each clinical site. We automate critical processes like inventory re-supply and expiry management to prevent stockouts and safeguard product integrity, with every transaction captured in a GxP-compliant audit trail. Below are the core features that make this possible.
The RTSM system is architected to manage complex, global supply chains. Administrators can create and manage multiple depots and multiple sites across different countries. The system provides a centralized view of inventory and logistics across the entire global study footprint.
The system proactively prevents stockouts using a configurable IP Threshold that automatically triggers re-supply orders when site inventory is low. Simultaneously, it manages product viability with automated expiry checks, sending timely email notifications for kits nearing their expiration date based on a customizable window.
Site users can generate manual shipment requests using the Stock Indent feature. When a shipment is processed, the randomisation and supply management system provides dedicated fields to capture all relevant shipment details, including the shipping agency, tracking number, and date of dispatch, ensuring a complete record of the transaction.
Provides complete control over the entire IMP supply chain, from ordering to returns. The RTSM system supports flexible ordering through both automated IP Thresholds and manual indents, while real-time tracking provides constant visibility into every kit's inventory status. Users can flag kits that have experienced a temperature excursion, and temperature data from loggers can be recorded upon receipt. The process concludes with a complete, multi-step returns workflow, featuring Project Manager approval gates to ensure full compliance and accountability.
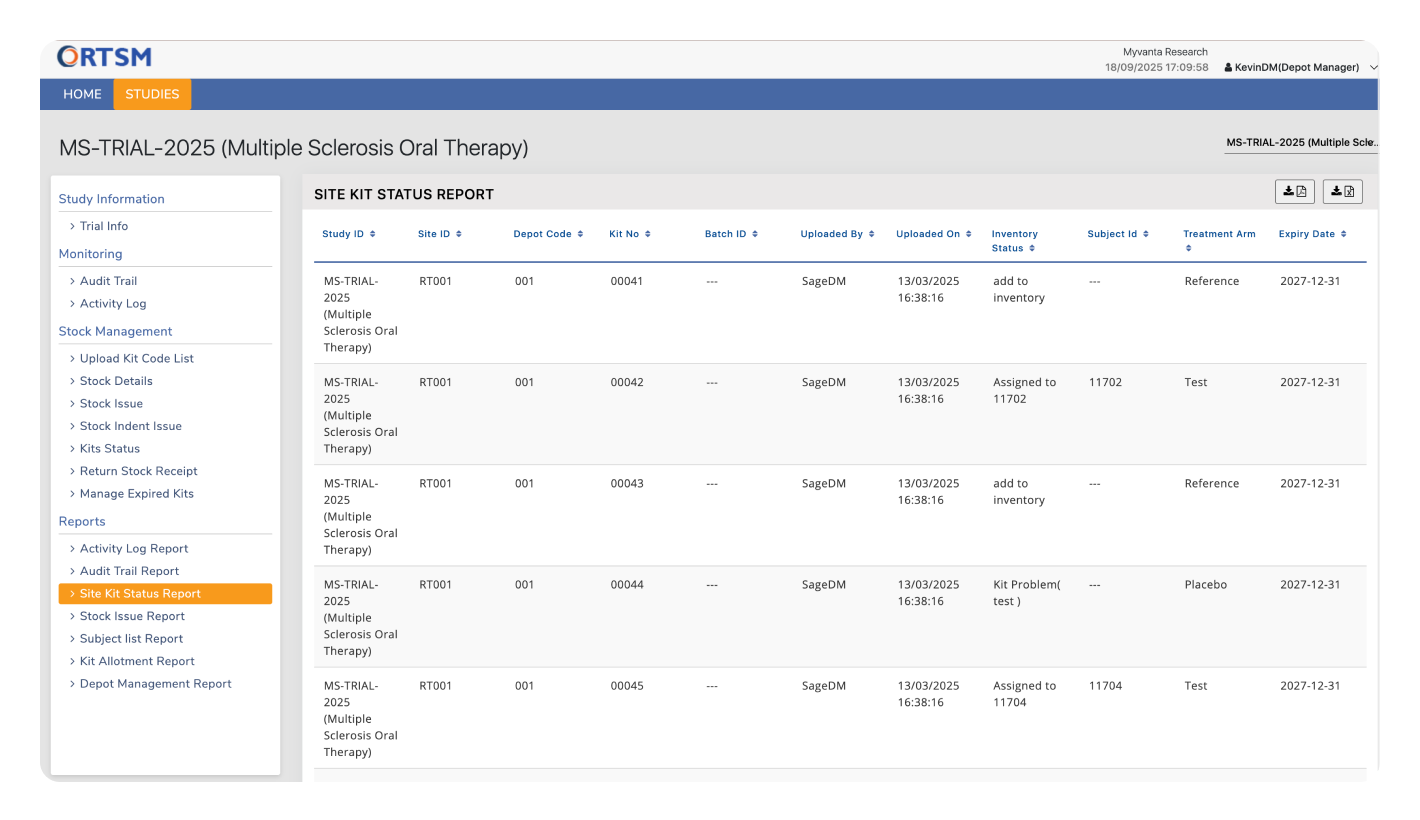
Provides robust global inventory tracking across depots and sites, offering full visibility into kit status, shipments, returns, and stock levels. This ensures efficient supply chain oversight from depot to site and supports timely resupply planning.
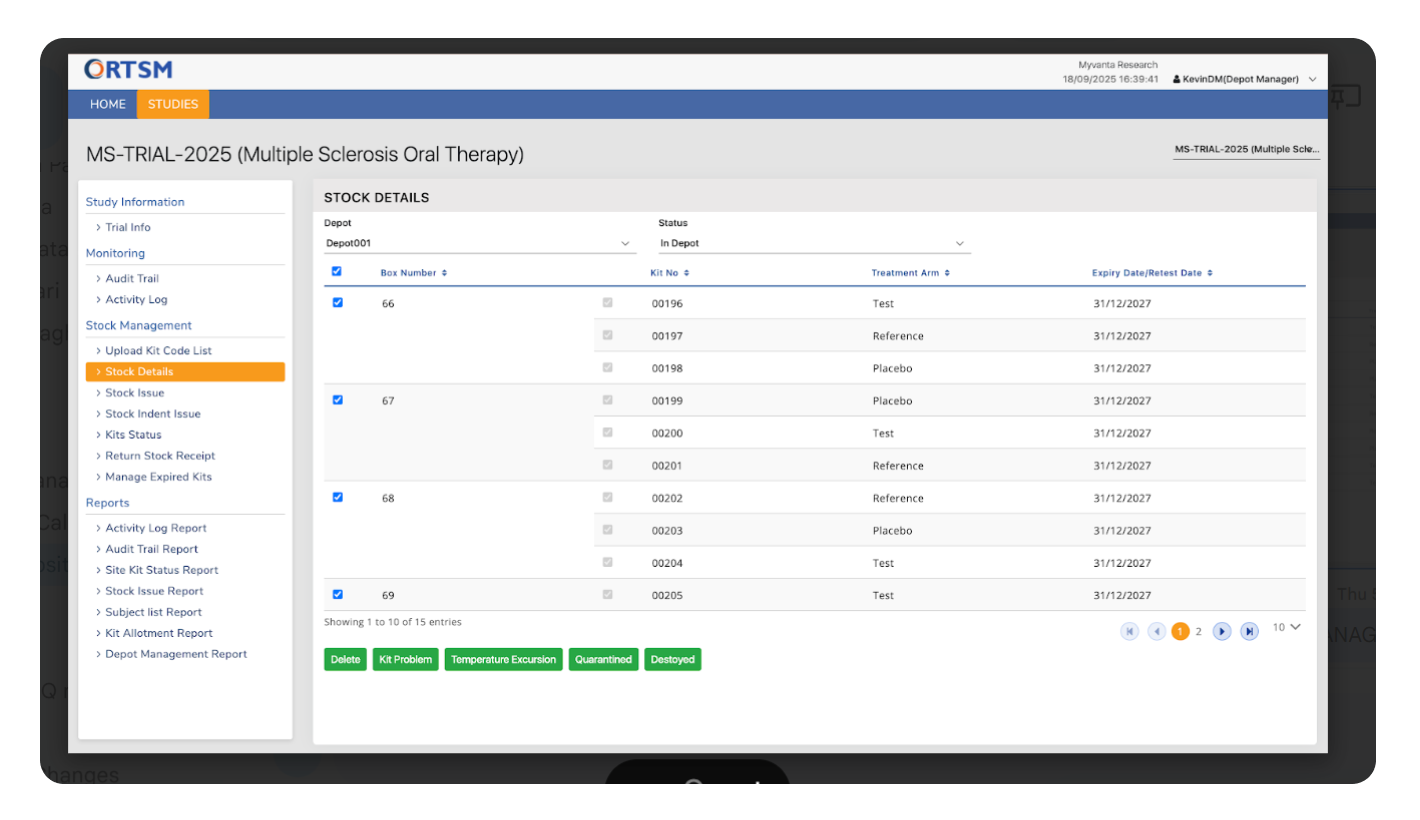
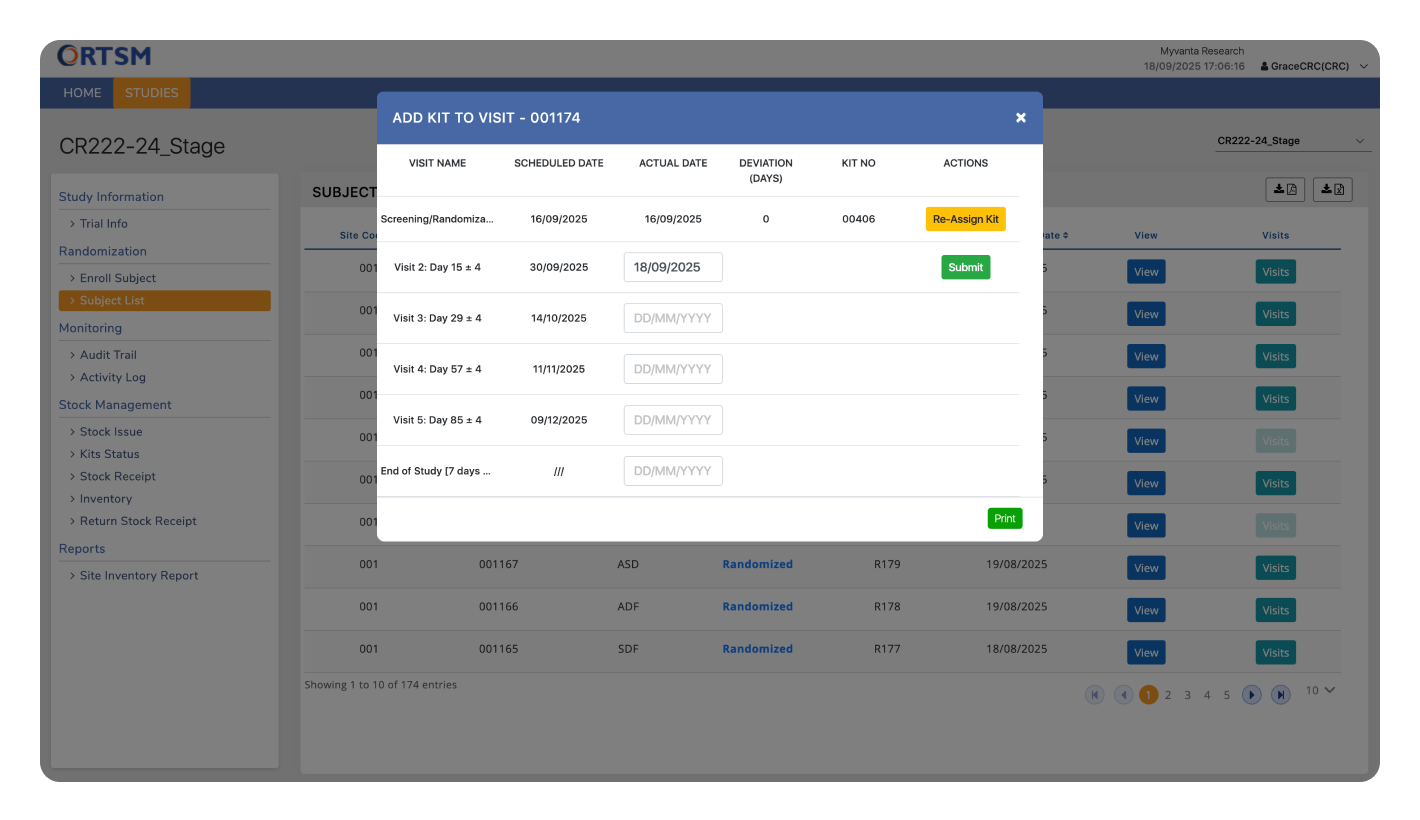
VISIT & DOSING MANAGEMENT
Deliver Accurate Dosing and Patient Safety at Every Visit
Our RTSM system ensures the right kit is assigned to the right subject at the right time by linking dispensing directly to the protocol-defined visit schedule. We automate the complex dosing logic, including adjustments for missed visits, to eliminate dispensing errors and ensure patient safety. All visits and dosing activities are captured in a complete audit trail, providing a clear and compliant record of protocol adherence. Below are the core features that make this possible.
The visit schedule, including permissible windows for each visit, can be configured to vary by cohort or other stratification factors as required by the protocol.
Once a visit is validated and an authorized user initiates the assignment, the RTSM system instantly allocates an appropriate kit from the site's available inventory. The assignment is immediately reflected in the inventory records and the subject's history. Subject receives the kit in real time at the site from the site inventory.
Kit assignment logic is tied to the completed visit. If a visit is missed, the associated kit assignment is skipped, ensuring subjects receive the correct kit for the next completed visit in their schedule.
The visit schedule can be updated mid-study within the integrated EDC, and the changes are reflected in the RTSM. The logic that determines which kit is assigned at each visit can also be modified to align with a protocol amendment, ensuring the trial can adapt without disruption.
REPORTING, ANALYTICS & AUDIT TRAIL
Access Inspection-Ready Reports and Compliant Data Instantly
Instantly access a comprehensive suite of audit-ready reports directly from the interface for real-time trial oversight. Our reports are exportable to PDF and Excel and built on a CDISC-compliant data structure, ensuring your data is always ready for analysis and regulatory submission.
Generate a complete, unchangeable history of all trial activities with a single click. Our RTSM provides dedicated, time-stamped reports for the Audit Trail, User Activity, and Unblinding events, giving you the transparent, traceable documentation required to be inspection-ready at all times.
Maintain complete control over your trial with on-demand access to live operational data. Monitor global inventory levels and track site performance in real-time with reports like the Depot Management, Site Inventory, and Subject List, enabling proactive, data-driven decision-making.
FAQS
Frequently Asked Questions
Discover quick solutions to your Clinion RTSM platform queries
Clinion RTSM software streamlines randomization for blinded, adaptive, and multicenter trials using automated, rule-based patient assignments. Its intuitive interface reduces setup time and complexity, enabling faster site activation and smoother execution across all trial phases.
Yes, Clinion RTSM automates inventory tracking, shipment planning, and site resupply across global sites. It provides real-time visibility and reduces drug wastage by up to 30%, making it ideal for managing supply logistics in multicenter studies.
Clinion RTSM delivers real-time analytics through an intuitive dashboard, enabling faster, data-driven decisions. Sponsors, CROs, and site teams gain instant visibility into randomization and supply status, improving oversight and operational efficiency.
Yes, Clinion RTSM enables secure, role-based emergency unblinding with full audit trails. The automated process protects patient safety and ensures quick action without compromising trial integrity.
Absolutely. Clinion RTSM supports streamlined batch data loading and can be deployed within 2–3 days. It’s AI-powered automation ensures fast, smooth trial setup, avoiding delays often caused by slow or complex systems.
Clinion RTSM meets global regulatory standards including 21 CFR Part 11, ICH GCP, and GDPR. It ensures secure access, detailed audit trails, and compliant data handling for both randomization and supply management.
Clinion RTSM is built for flexibility, allowing real-time updates to randomization and supply strategies without downtime. It ensures adaptive changes are applied instantly, keeping trials on track with no disruption.
Still have questions?
Explore how Clinion AI can accelerate your trial – reach out to our team.
Responsible AI
Clinion follows Responsible AI principles, ensuring its AI tools are built for safety and reliability, and remains committed to Data Privacy and Security at every step.
- Accountability
- Transparency
- Privacy & Security
- Reliability & Safety
- Fairness
Unlock the Future of Clinical Trials with Clinion.
Cut your trial costs by 35% and accelerate your time-to-market by 30%
Compliance
Fully Compliant with Global Standards


